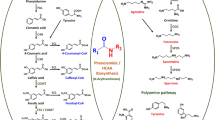
Abstract
Key message
We cloned two squalene epoxidases and five oxidosqualene cyclases, and identified their function using CRISPR/Cas9 tool and yeast heterologous expression.
Abstract
Triterpenes are the main active ingredients of Tripterygium wilfordii Hook.f., a traditional Chinese medicinal plant with many encouraging preclinical applications. However, the biosynthetic pathways of triterpenes in this plant are poorly understood. Here, we report on the isolation and identification of two squalene epoxidases (SQE6 and SQE7) and five oxidosqualene cyclases (OSC4-8) from T. wilfordii. Yeast complementation assays showed that TwSQE6 and TwSQE7 can functionally complement an erg1 yeast mutant that was constructed using the CRISPR/Cas9 system. The putative OSC genes were functionally characterized by heterologous expression in yeast. GC/MS analysis of the fermentation products of the transgenic yeast showed that both TwOSC4 and TwOSC6 are cycloartenol synthases, while TwOSC8 is a β-amyrin synthase. The discovery of these genes expands our knowledge of key enzymes in triterpenoid biosynthesis, and provides additional target genes for increasing the production of triterpenes in T. wilfordii tissue cultures by disrupting competing pathways, or in chassis cells by reconstituting the triterpenoid biosynthetic pathway.






Similar content being viewed by others
References
Abe I (2007) Enzymatic synthesis of cyclic triterpenes. Nat Prod Rep 24:1311–1331
Abe I, Prestwich GD (1995) Molecular cloning, characterization, and functional expression of rat oxidosqualene cyclase cDNA. Proc Natl Acad Sci USA 92:9274–9278
Almeida A, Dong L, Khakimov B, Bassard JE, Moses T, Lota F, Goossens A, Appendino G, Bak S (2018) A single oxidosqualene cyclase produces the Seco-triterpenoid α-Onocerin. Plant Physiol 176:1469–1484
Brendolise C, Yauk YK, Eberhard ED, Wang M, Chagne D, Andre C, Greenwood DR, Beuning LL (2011) An unusual plant triterpene synthase with predominant α-amyrin-producing activity identified by characterizing oxidosqualene cyclases from Malus × domestica. FEBS J 278:2485–2499
Chen Y, Gong Z, Chen X, Tang L, Zhao X, Yuan Q, Cai G (2013) Tripterygium wilfordii Hook F (a traditional Chinese medicine) for primary nephrotic syndrome. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008568.pub2
Cheng QQ, Tong YR, Wang ZH, Su P, Gao W, Huang LQ (2017) Molecular cloning and functional identification of a cDNA encoding 4-hydroxy-3-methylbut-2-enyl diphosphate reductase from Tripterygium wilfordii. Acta Pharm Sin B 7:208–214
Dong L, Pollier J, Bassard JE, Ntallas G, Almeida A, Lazaridi E, Khakimov B, Arendt P, de Oliveira LS, Lota F, Goossens A, Michoux F, Bak S (2018) Co-expression of squalene epoxidases with triterpene cyclases boosts production of triterpenoids in plants and yeast. Metab Eng 49:1–12
Dym O, Eisenberg D (2001) Sequence-structure analysis of FAD-containing proteins. Protein Sci 10:1712–1728
Gao C, Lou LL, Wang D, Zhang Y, Huang XX, Song SJ (2017) Chemical constituents from the roots of Tripterygium wilfordii and their cytotoxic activity. J Asian Nat Prod Res 19:725–731
Guo H, Li R, Liu S, Zhao N, Han S, Lu M, Liu X, Xia X (2016) Molecular characterization, expression, and regulation of Gynostemma pentaphyllum squalene epoxidase gene 1. Plant Physiol Biochem 109:230–239
Habtemariam S (2019) Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid Med Cell Longev 2019:8512048
Han JY, In JG, Kwon YS, Choi YE (2010) Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry 71:36–46
Han JY, Jo HJ, Kwon EK, Choi YE (2019) Cloning and characterization of oxidosqualene cyclases involved in taraxasterol, taraxerol and bauerenol triterpene biosynthesis in Taraxacum coreanum. Plant Cell Physiol 60:1595–1603
Howe E, Holton K, Nair S, Schlauch D, Sinha R, Quackenbush J (2010) MeV: MultiExperiment Viewer. In: Ochs MF, Casagrande JT, Davuluri RV (eds) Biomedical informatics for cancer research. Springer, Boston, pp 267–277
Jandrositz A, Turnowsky F, Hogenauer G (1991) The gene encoding squalene epoxidase from Saccharomyces cerevisiae: cloning and characterization. Gene 107:155–160
Johann S, Oliveira VL, Pizzolatti MG, Schripsema J, Braz-Filho R, Branco A, Smania A Jr (2007) Antimicrobial activity of wax and hexane extracts from Citrus spp. peels. Mem Inst Oswaldo Cruz 102:681–685
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Laranjeira S, Amorim-Silva V, Esteban A, Arro M, Ferrer A, Tavares RM, Botella MA, Rosado A, Azevedo H (2015) Arabidopsis squalene epoxidase 3 (SQE3) complements SQE1 and is important for embryo development and bulk squalene epoxidase activity. Mol Plant 8:1090–1102
Lee MH, Jeong JH, Seo JW, Shin CG, Kim YS, In JG, Yang DC, Yi JS, Choi YE (2004) Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol 45:976–984
Liu YJ, Zhao YJ, Zhang M, Su P, Wang XJ, Zhang XN, Gao W, Huang LQ (2014) Cloning and characterisation of the gene encoding 3-hydroxy-3-methylglutaryl-CoA synthase in Tripterygium wilfordii. Molecules 19:19696–19707
Lu L, Li F, Wang X (2010) Novel anti-inflammatory and neuroprotective agents for Parkinson’s disease. CNS Neurol Disord Drug Targets 9:232–240
Lu Y, Zhou J, Hu T, Zhang Y, Su P, Wang J, Gao W, Huang L (2018) A multifunctional oxidosqualene cyclase from Tripterygium regelii that produces both α- and β-amyrin. RSC Adv 8:23516–23521
Mandal A, Das V, Ghosh P, Ghosh S (2015) Anti-diabetic effect of friedelan triterpenoids in streptozotocin induced diabetic rat. Nat Prod Commun 10:1683–1686
Moses T, Pollier J, Almagro L, Buyst D, Van Montagu M, Pedreno MA, Martins JC, Thevelein JM, Goossens A (2014) Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiae using a C-16α hydroxylase from Bupleurum falcatum. Proc Natl Acad Sci USA 111:1634–1639
Pose D, Castanedo I, Borsani O, Nieto B, Rosado A, Taconnat L, Ferrer A, Dolan L, Valpuesta V, Botella MA (2009) Identification of the Arabidopsis dry2/sqe1-5 mutant reveals a central role for sterols in drought tolerance and regulation of reactive oxygen species. Plant J 59:63–76
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Rasbery JM, Shan H, LeClair RJ, Norman M, Matsuda SPT, Bartel B (2007) Arabidopsis thaliana squalene epoxidase 1 is essential for root and seed development. J Biol Chem 282:17002–17013
Roohbakhsh A, Iranshahy M, Iranshahi M (2016) Glycyrrhetinic acid and its derivatives: anti-cancer and cancer chemopreventive properties, mechanisms of action and structure- cytotoxic activity relationship. Curr Med Chem 23:498–517
Ruckenstuhl C, Lang S, Poschenel A, Eidenberger A, Baral PK, Kohut P, Hapala I, Gruber K, Turnowsky F (2007) Characterization of squalene epoxidase of Saccharomyces cerevisiae by applying terbinafine-sensitive variants. Antimicrob Agents Chemother 51:275–284
Tao X, Lipsky PE (2000) The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum Dis Clin North Am 26:29–50
Unland K, Putter KM, Vorwerk K, van Deenen N, Twyman RM, Prufer D, Gronover CS (2018) Functional characterization of squalene synthase and squalene epoxidase in Taraxacum koksaghyz. Plant Direct 2:e00063
Wang X, Tian W, Li Y (2008) Development of an efficient protocol of RNA isolation from recalcitrant tree tissues. Mol Biotechnol 38:57–64
Wilson SA, Roberts SC (2012) Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J 10:249–268
Xu R, Fazio GC, Matsuda SP (2004) On the origins of triterpenoid skeletal diversity. Phytochemistry 65:261–291
Xue Z, Duan L, Liu D, Guo J, Ge S, Dicks J, Om P, Osbourn A, Qi X (2012) Divergent evolution of oxidosqualene cyclases in plants. New Phytol 193:1022–1038
Zhang M, Su P, Zhou YJ, Wang XJ, Zhao YJ, Liu YJ, Tong YR, Hu TY, Huang LQ, Gao W (2015) Identification of geranylgeranyl diphosphate synthase genes from Tripterygium wilfordii. Plant Cell Rep 34:2179–2188
Zhou YJ, Gao W, Rong Q, Jin G, Chu H, Liu W, Yang W, Zhu Z, Li G, Zhu G, Huang L, Zhao ZK (2012) Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J Am Chem Soc 134:3234–3241
Zhou J, Zhang Y, Hu T, Su P, Zhang Y, Liu Y, Huang L, Gao W (2018) Functional characterization of squalene epoxidase genes in the medicinal plant Tripterygium wilfordii. Int J Biol Macromol 120:203–212
Zhou J, Hu T, Gao L, Su P, Zhang Y, Zhao Y, Chen S, Tu L, Song Y, Wang X, Huang L, Gao W (2019) Friedelane-type triterpene cyclase in celastrol biosynthesis from Tripterygium wilfordii and its application for triterpenes biosynthesis in yeast. New Phytol 223:722–735
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81773830 and no. 81973418), Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201710025022), the Support Project for High-level Teachers in Beijing Municipal Universities in the Period of the 13th Five-year Plan (CIT&TCD20170324) to W.G., the Key Project at central government level: the ability establishment of sustainable use for valuable Chinese medicine resources (2060302-1806-03).
Author information
Authors and Affiliations
Contributions
YL(Liu) and WG, LH designed this study; YL, JZ, TH, YL(Lu) carried out the experiments and performed results analysis; LG, LT, JG interpreted the data; YL(Liu) and JZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No competing interests were declared by the authors.
Additional information
Communicated by Chun-Hai Dong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhou, J., Hu, T. et al. Identification and functional characterization of squalene epoxidases and oxidosqualene cyclases from Tripterygium wilfordii. Plant Cell Rep 39, 409–418 (2020). https://doi.org/10.1007/s00299-019-02499-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02499-7