Abstract
Key message
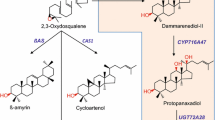
Protopanaxadiol (PPD) is an aglycone of dammarene-type ginsenoside and has high medicinal values. In this work, we reported the PPD production in transgenic tobacco co-overexpressing PgDDS and CYP716A47.
Abstract
PPD is an aglycone of ginsenosides produced by Panax species and has a wide range of pharmacological activities. PPD is synthesized via the hydroxylation of dammarenediol-II (DD) by CYP716A47 enzyme. Here, we established a PPD production system via cell suspension culture of transgenic tobacco co-overexpressing the genes for PgDDS and CYP716A47. The concentration of PPD in transgenic tobacco leaves was 2.3–5.7 µg/g dry weight (DW), depending on the transgenic line. Leaf segments were cultured on medium with various types of hormones to induce callus. Auxin treatment, particularly 2,4-D, strongly enhanced the production of DD (783.8 µg g−1 DW) and PPD (125.9 µg g−1 DW). Treatment with 2,4-D enhanced the transcription of the HMG-Co reductase (HMGR) and squalene epoxidase genes. PPD production reached 166.9 and 980.9 µg g−1 DW in a 250-ml shake flask culture and in 5-l airlift bioreactor culture, respectively.






Similar content being viewed by others
Abbreviations
- BAR:
-
Basta
- DD:
-
Dammarenediol-II
- PPD:
-
Protopanaxadiol
- DDS:
-
Dammarenediol-II synthase
- PgDDS:
-
Panax ginseng dammarenediol-II synthase
- CYP716A47:
-
Cytochrome P450 716A47
- qPCR:
-
Real-time polymerase chain reaction
References
Bae EA, Han MJ, Kim EJ, Kim DH (2004) Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res 27:61–67
Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C (1995) Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol 109:1337–1343
Corey EJ, Matsuda SP, Bartel B (1993) Isolation of an Arabidopsis thaliana gene encoding cycloartenol synthase by functional expression in a yeast mutant lacking lanosterol synthase by the use of a chromatographic screen. Proc Natl Acad Sci USA 90:11628–11632
Dai Z, Liu Y, Zhang X, Shi M, Wang B, Wang D, Huang L, Zhang X (2013) Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng 20:146–156
Du GJ, Dai Q, Williams S, Wang CZ, Yuan CS (2011) Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities. Anticancer Drugs 22:35–45
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol 198:16–32
Durst F, Nelson DR (1995) Diversity and evolution of plant P450 and P450-reductases. Drug Metabol Drug Interact 12:189–206
Han JY, Kwon YS, Yang DC, Jung YR, Choi YE (2006) Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol 47:1653–1662
Han JY, Kim HJ, Kwon YS, Choi YE (2011) The cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 52:2062–2073
Han JY, Wang HY, Choi YE (2014) Production of dammarenediol-II triterpene in a cell suspension culture of transgenic tobacco. Plant Cell Rep 33:225–233
Hasegawa H, Sung JH, Benno Y (1997) Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med 63:436–440
Jia W, Yan H, Bu X, Liu G, Zhao Y (2004) Aglycone protopanaxadiol, a ginseng saponin, inhibits P-glycoprotein and sensitizes chemotherapy drugs on multidrug resistant cancer cells. ASCO annual meeting proceedings (post-meeting edition). J Clin Oncol 22(Suppl):9663
Karikura M, Miyase T, Tanizawa H, Taniyama T, Takino Y (1991) Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of rats. Chem Pharm Bull 39:2357–2361
Lee MH, Jeong JH, Seo JW, Shin CG, Kim YS, In JG, Yang DC, Yi JS, Choi YE (2004) Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol 45:976–984
Leung KW, Wong AS (2010) Pharmacology of ginsenosides: a literature review. Chin Med 5:20
Li G, Wang Z, Sun Y, Liu K, Wang Z (2006) Ginsenoside 20(S)-protopanaxadiol inhibits the proliferation and invasion of human fibrosarcoma HT1080 Cells. Basic Clin Pharmacol Toxicol 98:588–592
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the \(2^{{ - \varDelta \varDelta C_{\text{T}} }}\) Method. Methods 25:402-408
Lu AY, Junk KW, Coon MJ (1969) Resolution of the cytochrome P450 containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem 244:3714–3721
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Georgiev MI, Kim YS, Jeong CS, Kim SJ, Park SY, Paek KY (2014) Ginsenoside: prospective for sustainable biotechnolotgical production. Appl Microbiol Biotechnol 98:6243–6254
Musende AG, Eberding A, Wood CA, Adomat H, Fazli L, Hurtado-Coll A, Jia W, Bally MB, Tomlinson Guns ES (2012) A novel oral dosage formulation of the ginsenoside aglycone protopanaxadiol exhibits therapeutic activity against a hormone-insensitive model of prostate cancer. Anticancer Drugs 23:543–552
PanaGin Pharmaceuticals Inc (2009) http://www.panagin.com. Accessed Oct 2009
Popovich DG, Kitts DD (2004) Ginsenosides 20(S)-protopanaxadiol and Rh2 reduce cell proliferation and increase sub-G1 cells in two cultured intestinal cell lines (Int-407 and Caco-2). Can J Physiol Pharmacol 82:183–190
Qi LW, Wang CZ, Yuan CS (2010) American ginseng: potential structure–function relationship in cancer chemoprevention. Biochem Pharmacol 80:947–954
Ro D, Ehlting J, Douglas C (2002) Cloning, functional expression, and subcellular localization of multiple NADPH-cytochrome P450 reductases from hybrid poplar. Plant Physiol 130:1837–1851
Ryder NS (1991) Squalene epoxidase as a target for the allylamines. Biochem Soc Trans 19:774–777
Saklani A, Kutty SK (2008) Plant-derived compounds in clinical trials. Drug Disc Today 13:161–171
Shibata S (2001) Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci 16:S28–S37
Tansakul P, Shibuya M, Kushiro T, Ebizuka Y (2006) Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett 580:5143–5149
Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M (2003) Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos 31:1065–1071
Tholl D (2006) Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol 9:1–8
Verpoorte R, Memelink J (2002) Engineering secondary metabolite production in plants. Curr Opin Biotech 13:181–187
Wu S, Chappell J (2008) Metabolic engineering of natural products in plants; tools of the trade and challenges for the future. Curr Opin Biotech 19:145–152
Zhu GY, Li YW, Tse AK, Hau DK, Leung CH, Yu ZL, Fong WF (2011) 20(S)-protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur J Pharmacol 668:88–98
Zou W, Yue P, Khuri FR, Sun SY (2008) Coupling of endoplasmic reticulum stress to CDDO-Me-induced up-regulation of death receptor 5 via a CHOP-dependent mechanism involving JNK activation. Cancer Res 68:7484–7492
Acknowledgments
This work was supported by the Rural Development Administration, Republic of Korea [Next-Generation BioGreen 21 Program (PJ011285)], and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2064352).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Sato.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chun, JH., Adhikari, P.B., Park, SB. et al. Production of the dammarene sapogenin (protopanaxadiol) in transgenic tobacco plants and cultured cells by heterologous expression of PgDDS and CYP716A47 . Plant Cell Rep 34, 1551–1560 (2015). https://doi.org/10.1007/s00299-015-1806-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1806-9