Abstract
To increase immune responses of plant-based vaccines in intestine mucosal immune systems, a synthetic neutralizing epitope (sCOE) gene of porcine epidemic diarrhea virus (PEDV) was fused with M cell-targeting ligand (Co1) and introduced into a plant expression vector under the control of rice amylase 3D promoter. The sCOE–Co1 fusion gene was introduced into rice calli via the particle bombardment-mediated transformation method. The stable integration and transcriptional expression of the sCOE–Co1 fusion gene was confirmed by genomic DNA PCR amplification and Northern blot analysis, respectively. The expression of the COE–Co1 fusion protein was confirmed by immunoblot analysis. The highest expression level of the COE–Co1 fusion protein reached 0.083 % of the total soluble protein according to quantitative densitometry of Western blot analysis. Mice immunized with transgenic rice calli protein extracts induced significant serum IgG and fecal IgA antibody levels against purified bacterial COE. The systemic and mucosal immune responses were confirmed by measuring COE-specific IgG and IgA antibody-secreting cells in the lymphocytes extracted from the spleen and COE-specific IgA antibody-secreting cells in the Peyer’s patches from immunized mice. These results indicated that oral immunization of plant-produced COE–Co1 fusion protein could elicit efficient systemic and mucosal immune responses against the COE antigen.
Key message Neutralizing epitope from porcine epidemic diarrhea virus-M cell targeting ligand fusion protein was produced in transgenic rice calli and elicited systemic and mucosal immune responses by oral administration in mice.
Similar content being viewed by others
Introduction
Porcine epidemic diarrhea virus (PEDV) is classified as a member of Group I of the genus Coronaviruses, the family Coronaviridae and the order Nidovirales (Cavanagh and Britton 2008). PEDV is an etiologic agent of diarrhea in pigs, especially newborn pigs (Cavanagh 2005; Cavanagh et al. 1993). PEDV disrupts villus enterocytes and causes villious atrophy within the jejunum and ileum, leading to a mortality rate of up to 95 % in infected piglets (Ducatelle et al. 1981). The genome consists of a single molecule of linear positive-sense, single-stranded RNA. The complete sequence of the entire genome of strain CV777 was found to be 28,033 nucleotides in length excluding the poly A-tail (Kocherhans et al. 2001). The viral encoded-proteins from the PEDV genome have four structural proteins: a large spike glycoprotein or peplomer (S, 180–220 kDa), an integral membrane glycoprotein (M, 27–32 kDa), a small envelope protein with a small amount of virions, and a phosphorylated nucleocapsid protein (N, 55–58 kDa) (Cavanagh and Britton 2008; Egberink et al. 1988). Spike proteins attach viral particles to receptors on the host cells and subsequently penetrate into the cells by membrane fusion. The S glycoprotein also stimulates induction of neutralizing antibodies in the host (Duarte and Laude 1994; Yeo et al. 2003). The S glycoprotein is, therefore, essential for the development of a vaccine against PEDV. The predicted polypeptide was 1,383 amino acids long containing 29 potential N-linked glycosylation sites and showed structural features similar to those of the coronavirus spike protein (Duarte and Laude 1994). The S glycoprotein of PEDV lacks a proteolytic site to yield cleaved amino and carboxy subunits, S1 and S2, but can be divided into the S1 domain (1–789 amino acids) and the S2 domain (790–1,383 amino acids) based on the presence of the conserved nonamer and GxCx motifs at the proteolytic cleavage site of S protein in other members of coronavirus, Group II (Follis et al. 2006). Based on the sequence information for the neutralizing epitope of the transmissible gastroenteritis virus, Chang et al. identified the neutralizing epitope region of PEDV (CO-26 K equivalent, COE gene) as containing 139 amino acids spanning the region of 499–638 amino acids within the S1 domain (Chang et al. 2002). In previous studies, the synthetic COE (sCOE) gene of PEDV, which was modified based on the plant-optimized codon usage, was expressed in tobacco plants (Bae et al. 2003; Kang et al. 2004, 2005). The sCOE gene fused with an Escherichia coli heat-labile toxin B subunit (LTB) gene was expressed in transgenic tobacco plants (Kang et al. 2006), rice seeds (Oszvald et al. 2007) and lettuce plants (Huy et al. 2009). Alternatively, the sCOE gene fused with cholera toxin B subunit (CTB) was expressed in lettuce plants (Huy et al. 2011). The COE gene is, therefore, considered to be an important target in the development of subunit vaccine against PEDV infection.
Transgenic plants and transgenic cell suspension culture systems have been considered to be bioreactors for producing a variety of antigen proteins and promising production systems. However, the low expression level of antigens in transgenic plants induces low immune responses and immune tolerances in animal models. This limitation is an important bottleneck that stands in the way of developing plant-based edible vaccines. One solution is to increase antigen delivery into mucosal immune systems via ligands, such as cholera toxin B subunit (CTB), enterotoxigenic Escherichia coli heat-labile enterotoxin B subunit (LTB) and a variety of B subunits of toxins. Recently, Kim et al. (2010a) showed that the M cell-target peptide ligand, Co1, in orally treated mice enhanced the uptake of fused antigen into the effective sites of mucosal immune systems and immune responses against fused antigen as compared to antigens alone.
Inducible rice α-amylase 3D promoter (RAmy3D) was used to improve the efficiency of expression of target genes in rice cell suspension culture systems under sugar starvation conditions. The RAmy3D gene is first expressed during rice seedling development. The RAmy3D promoter contains cis elements, including the amylase element (TATCCAT), the CGACG element, and a G box-related element (CTACGTGGCCA). These elements are needed for high-level expression of RAmy3D under conditions of sugar starvation (Hwang et al. 1988). The RAmy3D promoter has been used to highly express heterologous genes in rice cell suspension culture (Kim et al. 2008a, b; Hong et al. 2006).
In the present study, a synthetic neutralizing epitope from PEDV was fused with the M cell-target peptide ligand, Co1, to produce effective plant-derived edible vaccine against PEDV and was introduced into rice calli via the particle bombardment-mediated transformation method. The expression of the COE–Co1 fusion protein was confirmed by Western blot analysis. The immunogenicity of rice callus-based COE–Co1 fusion protein was confirmed in mice via oral administration.
Materials and methods
Construction of rice expression vector
The sCOE–Co1 fusion gene was amplified from plasmid pMYV93 containing the sCOE gene (Kang et al. 2005) as a template for polymerase chain reaction (PCR) amplification with forward primer (5′-GGA TCC GTT ACT TTG CCA TCC TT-3′) and reverse primer (5′-GGT ACC TGG AAG TGG AGA TCT AGC TGG AAG CTG ATG AAA AGA AAC ATC TGT GAT TCC C-3′) in which the Co1 ligand was included in the reverse primer (shown above in italics) (Kim et al. 2010a). BamHI and KpnI restriction enzyme sites (underlined in primers) were included in primers for convenient subcloning. The PCR reaction was conducted with the following PCR condition: 1 cycle at 94 °C for 5 min; 30 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; 1 cycle at 72 °C for 5 min. PCR products were cloned into the pGEM®T-Easy vector (Promega, Madison, WI) to create pMYV730. Its sequence was confirmed via DNA sequence analysis with T7 and SP6 primers. The sCOE–Co1 fusion gene from pMYV730 was digested with BamHI and KpnI and introduced into the same sites of the rice expression vector pMYV657 under the control of the RAmy3D promoter expression system with a signal peptide and the 3′-untranslated region (3′-UTR) of the Ramy3D gene (Kim et al. 2012) to form pMYV733.
Rice transformation
The dehusked mature rice grains (Oryza sativa L. cv. Dongin) were sterilized and cultured on N6 salts and vitamins (Chu et al. 1975) supplemented with 2,4-dichlorophenoxyalic acid (2,4-D) (2 mg/l), sucrose (30 g/l), and kinetin (0.2 mg/l) for 7 days to assure callus induction. Rice calli were prepared and transformed with plasmid pMYV733 via the particle bombardment-mediated transformation method (Chen et al. 1998). After bombardment, calli were cultured on N6 co-culture medium supplemented with 2,4-D (2 mg/l), sucrose (30 g/l), kinetin (0.2 mg/l), and glucose (10 g/l) without antibiotics for 3 days in dark conditions, then transferred to N6 selection medium (N6SE) supplemented with antibiotic hygromycin B (50 mg/l) for selection. After 2–3 weeks, putative transgenic rice calli were observed.
Genomic DNA PCR analysis
Genomic DNA was isolated from wild type and putative transgenic rice calli using a ZymoBead™ Genomic DNA Kit (Zymo Research, Orange, CA). The concentration of genomic DNA was measured at 260 nm using an ultraviolet (UV) spectrophotometer. PCR was carried out in a 25 μl volume containing 500 ng of genomic DNA, sCOE–Co1 fusion gene-specific primers (forward primer 5′-GGA TCC GTT ACT TTG CCA TCC TT-3′ and reverse primer 5′-GGT ACC TGG AAG TGG AGA TCT AGC-3′), 200 μM dNTPs, 1 × Taq polymerase buffer, and 2 U iTaq polymerase (iNtRON Biotechnology, Seoul, Korea). PCR products were analyzed by 1.0 % agarose gel electrophoresis followed by staining in ethidium bromide solution.
Northern blot analysis
The COE–Co1 fusion genes under the control of the RAmy3D promoter in transgenic rice calli were induced for 5 days under sugar starvation conditions. Total RNA was extracted from wild-type and transgenic rice calli using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to the supplier’s protocol. The RNA samples (30 μg) were separated by electrophoresis on 1.2 % formaldehyde–agarose gel (Lehrach et al. 1977) and capillary-blotted onto Hybond N+ membrane (Amersham Pharmacia Biotech, Piscataway, NJ). The blot was hybridized overnight in a Hybridization Incubator (Finemould Precision Ind. Co., Seoul, Korea) with a ³²P-labeled, randomly primed sCOE-Co1 probe (Promega) at 65 °C in a buffer (pH 7.4) containing 1 mM EDTA, 250 mM Na2HPO4.7H2O, 1 % hydrolyzed casein, and 7 % SDS. The blot was washed twice with washing buffer A (2 × SSC, 0.1 % SDS), twice with washing buffer B (2 × SSC, 1 % SDS) and twice with washing buffer C (0.1 × SSC, 0.1 % SDS) for 15 min each at 65 °C. Hybridized bands were detected by autoradiography using X-ray film (AGFA, Mortsel, Belgium).
Immunoblot detection
Transgenic rice calli were analyzed to detect the presence of COE–Co1 fusion protein by immunoblot analysis. The expression of the COE–Co1 fusion gene was induced in an N6 liquid medium without sucrose. Total soluble protein was extracted from the rice calli by grinding in liquid nitrogen with a mortar and pestle. The homogenate was suspended with a protein extraction buffer (200 mM Tris–Cl, pH 8.0, 100 mM NaCl, 400 mM sucrose, 10 mM EDTA, 14 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, and 0.05 % Tween 20) and centrifuged at 13,000 g for 15 min at 4 °C to remove insoluble cell debris. The protein concentration was measured by the Bradford protein assay (Bio-Rad Inc., Hercule, CA). An aliquot of protein extracts containing 80 μg of total soluble protein was separated by 12 % sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad) at 120 V for 2–3 h in a Tris–glycine buffer at a pH of 8.3 (25 mM Tris–Cl, 250 mM glycine and 0.1 % SDS). The separated protein bands were transferred onto Hybond C membranes (Amersham Pharmacia Biotech) in transfer buffer (50 mM Tris–Cl, 40 mM glycine, and 20 % methanol) using a mini-transblot apparatus (Bio-Rad) at 130 mA for 3 h. Non-specific antibody reactions were blocked with 10 % non-fat milk powder in TBST buffer (Tris-buffered saline with 0.05 % Tween 20). Membranes were incubated with a 1:5,000 dilution of mouse anti-COE polyclonal antibodies in a TBST antibody dilution buffer containing 3.0 % non-fat dry milk. Membranes were then washed three times with the TBST buffer and incubated for 2 h with a 1:7,000 dilution of anti-mouse IgG conjugated with alkaline phosphatase (Promega S372B) in a TBST buffer. Membranes were washed twice with the TBST buffer and once with a TMN buffer (100 mM Tris–Cl, pH 9.5, 5 mM MgCl2, and 100 mM NaCl). Color was developed using BCIP/NBT solution (Sigma, St. Louis, MO). The membranes were subjected to densitometry (Alpha Ease FC™ software Version 3.3.3, AlphaInotech) to estimate the protein expression levels.
PNGase F and endoglycosidase (Endo F1 and Endo H) treatment
N-glycosylated structures of COE protein produced in transgenic rice calli were determined using deglycosylation enzymes. The transgenic rice calli were ground as described above. The homogenate was suspended with denaturing extract buffer (including 0.5 % SDS and 1 % 2-mercaptoethanol) and subsequently centrifuged at 13,000 g for 15 min at 4 °C to remove insoluble cell debris. The protein extracts were boiled for 10 min and chilled on ice for 5 min. The N-glycosylation products of plant-produced protein were removed using PNGase F and endoglycosidases F1 and H (Endo F1 and Endo H) (ELPIS Biotech, Seoul, Korea) in 35 μl of reaction mix including 3.5 μl of 10 × reaction buffer, 1,000 units of enzyme and 50 μg of protein extract for 1 h at 37 °C. The treatments were visualized using Western blot analysis.
Preparation of rice calli protein extract
Transgenic rice calli with high expression level (line #9) were cultured in an N6 liquid medium, and the production of COE–Co1 fusion protein was induced under a sugar starvation condition. The transgenic rice calli were collected using a vacuum-pump filter and crushed using a blender. Total soluble protein was extracted with a phosphate buffered saline (PBS) buffer and centrifuged at 13,000 g for 15 min at 4 °C to remove insoluble cell debris. Total soluble protein was precipitated by ammonium sulfate with appropriate concentration (Coligan 1996). The precipitated proteins were dialyzed in PBS buffer to remove ammonium sulfate prior to being fed to the mice. After dialysis, the recombinant proteins were analyzed using SDS-PAGE, and the level of heterologous protein was estimated using quantitative densitometry of Western blot analysis.
Oral immunization with rice protein extract
Female BALB/c mice were purchased from Dong Yang Oriental Company (Seoul, Korea) and randomly divided into two groups with five mice each. Experimental mice were administered through gavage every week for 10 weeks (Kong et al. 2001; Yoshida et al. 2011), as shown in Fig. 5. Prior to receiving the plant protein extracts, the mice fasted for 8 h and were gavaged with 2.0 ml of PBS buffer at a pH of 7.0 containing rice callus protein extract (approximately 40 μg of COE–Co1 fusion protein). Blood and fecal samples were collected 10 days after feeding at weeks 4, 6, 8, and 10, as previously described (Jespersgaard et al. 1999). The collected blood samples were stored at room temperature for 1 h and chilled on ice for 1 h before centrifugation with 13,000 g for 10 min. The serum was collected from the supernatant and stored at −70 °C for further assays. Fecal samples (200 mg) were dissolved in 1 ml of PBS buffer containing 0.01 % sodium azide. Insoluble materials were removed by centrifugation at 13,000 g for 10 min. Fecal extracts were also stored at −70 °C for further analysis.
Detection of antibody in sera and fecal extracts
For analysis of antigen-specific IgG and IgA antibodies, ELISA was conducted according to a previously described protocol (Kim et al. 2010b) with minor modifications. Briefly, the ELISA plates were coated with 100 μl of antigen (0.8 μg/well) dissolved in a bicarbonate buffer (15 mM Na2CO3, 25 mM NaHCO3, pH 9.6), covered with plastic wrap and incubated at 4 °C overnight. The wells were washed three times with PBST buffer (PBS plus 0.05 % Tween-20), blocked by the addition of 300 μl per well of 1 % BSA in a PBS buffer, incubated at 37 °C for 2 h, and followed by three washes with PBST buffer. The sera and fecal extracts were diluted to 1:100 or 1:4 with PBS buffer, loaded into the antigen-coated plates and incubated at 37 °C for 2 h. The plates were washed three times with PBST buffer, incubated with 100 μl of 1:10,000 dilution of anti-mouse IgG (Sigma A-3688) or anti-mouse IgA (Sigma A-4937), conjugated with alkaline phosphatase for 2 h at 37 °C, and washed three times with PBST buffer. The plates were developed with the addition of 100 μl of alkaline phosphatase buffer [10 % (v/v) diethanol amine, 0.1 % MgCl2, 0.02 % sodium azide, pH 9.8] plus one tablet of phosphate substrate (Sigma S0942-100TAB) for 30 min at room temperature in dark conditions. The plates were read at a 405 nm wavelength in an ELISA reader (Packard Instrument, Meriden, CA).
ELISPOT assays
Lymphocytes from the spleen and Peyer’s patches (PPs) of immunized mice showing the highest COE-specific IgG antibody levels were isolated at day 10 after a final booster, as described by Kim et al. (2010a). ELISPOT assays were conducted to measure the number of COE-specific antibody-secreting cells (ASCs) using the lymphocytes isolated from the spleen and PPs, as also described by Kim et al. (2010a).
Data analysis
The results were calculated using Excel 2007 software (Microsoft) and expressed as mean ± standard deviation of at least three independent experiments. Statistical significance was calculated using Student’s t test, and p < 0.05 were considered statistically significant.
Results
Construction of the rice expression vector and rice transformation
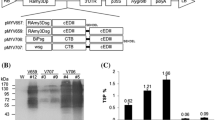
The synthetic COE (sCOE) gene was fused with the M cell-target peptide ligand, Co1, at the 3′-end (sCOE-Co1) and introduced into the rice expression vector under the control of RAmy3D promoter expression system, yielding plasmid pMYV733 (Fig. 1a). The plant expression vector was transformed into rice calli via the particle bombardment-mediated transformation method. After 2–3 weeks, the putative transgenic calli appeared on the selection media containing hygromycin B as the selection marker. The PCR products corresponding in size to the sCOE–Co1 fusion gene were amplified by genomic DNA PCR amplification analysis in 14 putative transgenic rice calli. All transgenic rice calli showed an expected band with 468 bp (Fig. 1b). The DNA band was not amplified in the wild-type plant genomic DNA.
Rice expression vector pMYV733 and genomic DNA PCR analysis. The synthetic COE gene (sCOE) was fused with the M cell-target peptide ligand, Co1, introduced into a plant expression vector under the control of a signal peptide (3Dsp), promoter (RAmy3D-p), and untranslated region (3′-UTR) of a rice amylase 3D gene, and transformed into rice calli. The hygromycin phosphotransferase (HPT) gene was used as a selection marker under the control of Cauliflower mosaic virus 35S promoter (35S-p) and terminator (35S-t). LB and RB are left and right border of T-DNA, respectively (a). Genomic DNA PCR amplification was conducted to amplify the sCOE–Co1 fusion gene in genomic DNA of wild-type and putative transgenic rice calli (b). Lane M is a 100 base-pair DNA ladder (ELPIS Biotech, Seoul, Korea). Lane PC is PCR products amplified from plasmid pMYV733 used as a positive control. Lane NC is a wild type used as a negative control. Lanes 1–14 are independent putative transgenic lines
Expression of COE–Co1 in transgenic rice calli
The expression of the sCOE–Co1 fusion gene under the control of inducible RAmy3D promoter in transgenic rice calli was induced for 5 days on solid N6 media without sucrose. The mRNA transcripts of the sCOE–Co1 fusion gene were identified by Northern blot analysis using a 32P-labeled sCOE-Co1 fusion gene probe. Nine of 12 transgenic rice calli lines showed the presence of the sCOE–Co1 transcripts (Fig. 2). Two transgenic lines (lines #9 and #11) showing strong mRNA expression were selected for Western blot analysis.
Northern blot analysis to determine the expression of the sCOE–Co1 fusion gene. Total RNA (30 μg) prepared from wild type and transformed rice calli were hybridized with 32P-labeled sCOE–Co1 fusion gene probe. Lane NC is total RNA from wild-type rice calli. Lanes 1–12 are total RNA from transgenic rice calli
The expression of the COE–Co1 fusion protein was induced on a time course under the condition of sucrose starvation in two transgenic lines (lines #9 and #11). Immunoblot analysis was conducted to detect the COE–Co1 fusion protein in transgenic rice calli protein extracts. The COE–Co1 fusion protein with a molecular weight less than 26 kDa was detected, while the bacterial COE positive control was identified around 16 kDa. The COE–Co1 fusion protein was detected at a larger size than expected due to two potential N-glycosylation sites. To analyze the N-glycosylation status, the protein extracts were treated with PNGase F, Endo F1 and Endo H. PNGase F removes the glycans from the core peptide in denatured proteins, and Endo F1 and Endo H cleave between the two N-acetylglucosamine residues in the diacetylchitobiose core of the oligosaccharide, generating a truncated sugar molecule with one N-acetylglucosamine residue. After treatment with the deglycosylation enzyme, protein bands smaller than the untreated band were detected (Fig. 3c), indicating that the rice-produced COE–Co1 fusion protein had undergone N-glycosylation.
Detection and N-glycosylation analysis of COE–Co1 fusion protein. Western blot analysis of COE–Co1 fusion protein in transgenic rice calli line #9 (a) and line #11 (b) with anti-COE antibody as the primary antibody. Lane M is prestained with SDS-PAGE standards (ELPIS Biotech). Lanes 10, 20 and 40 contain predetermined amounts (10, 20 and 40 ng) of COE protein purified in E. coli. Lane NC is protein extracts from wild-type rice calli. Lanes 1–5 are protein extracts from transgenic calli on 1–5 days after expression induction. Analysis of N-glycosylation of the COE–Co1 fusion protein (c). Lane N is protein extracts untreated with enzymes. Lanes F, F1 and H are protein extracts treated with enzymes PNGase F, Endo F1 and Endo H, respectively
Quantification of COE–Co1 fusion protein
The detected bands in Western blot analysis were scanned to estimate the expression level of COE–Co1 fusion protein using the densitometry method. Predetermined protein concentrations (10, 20 and 40 ng) were used to make a standard curve (Fig. 3a, b). The expression level of COE–Co1 fusion protein was estimated on the time course under the sugar starvation condition in transgenic line #9 (Fig. 4a) and line #11 (Fig. 4b). The highest expression level reached 0.083 % of TSP in transgenic rice calli in line #9 at day 3 after induction (Fig. 4a).
Serum IgG and fecal IgA antibody in orally immunized mouse
Experimental groups of BALB/c mice (n = 5) received transgenic or wild-type rice calli protein extracts orally on 10 separate occasions (Fig. 5a). Serum IgG antibody against bacterial COE in immunized mice with transgenic plant protein extracts containing COE–Co1 fusion protein were induced at a higher rate as compared to that observed in mice immunized with wild-type plant protein extracts at weeks 8 and 10 (Fig. 5b). The mouse immunized with COE–Co1 fusion protein induced stronger COE-specific IgA levels in comparison to the mice fed with wild-type rice protein extract as negative control at weeks 8 and 10 (Fig. 5c).
Mouse feeding strategy and detection of COE-specific antibody. Protein extracts of transgenic rice calli expressing COE–Co1 fusion protein and wild type were concentrated by precipitating with ammonium sulfate for mouse oral immunization. Each mouse in the experimental group (n = 5) was gavaged with 2 ml of PBS buffer containing 40 μg of COE–Co1 fusion protein in total of 10 time feeds with an interval of 1 week (a). Blood and fecal samples were collected 10 days after 4, 6, 8, and 10-week feedings. The mouse sera and fecal samples were diluted 100-fold and 4-fold, respectively, and used to measure serum IgG (b) and fecal IgA (c) antibody levels against bacterial COE. The representative results show the average OD results from five immunized mice. Error bars represent standard deviation
Measurement of COE-specific immune responses
To monitor the antigen-specific immune responses in systemic area and mucosal compartment, the numbers of COE-specific IgG- and IgA-secreting cells in lymphocytes isolated from the spleen and Peyer’s patches after the final oral immunization were evaluated by ELISPOT. The numbers of COE-specific IgG- and IgA-secreting cells in lymphocytes isolated from the spleen in immunized mice with COE–Co1 fusion protein were observed at threefold and eightfold higher levels, respectively, as compared to the mice immunized with wild-type plant proteins (Fig. 6a). The Peyer’s patches lymphocytes isolated from immunized mice contained twofold larger populations of cells secreting anti-COE IgA antibody as compared to the control mice (Fig. 6b). Collectively, oral immunization with plant-derived COE–Co1 fusion proteins was able to induce efficient systemic and mucosal immune responses against the COE antigen.
Measurement of COE-specific IgG and IgA antibody-secreting cells (ASCs) in immunized mice. The COE-specific ASCs per 105 SPLs (a) or per 106 PPLs (b) were determined using ELISPOT. SPL and PPL represent lymphocytes from the spleen and Peyer’s patches, respectively. Error bars represent standard deviations. *p < 0.05; **p < 0.01; ***p < 0.001 indicate significant differences between the values compared
Discussion
PEDV causes acute enteritis with a death rate greater than 95 % in infected piglets, leading to significant economic losses in swine husbandry (Kang et al. 2005). PED is one of the most devastating diseases in suckling piglets, and it frequently occurs in Korea (Lee and Yeo 2003). Therefore, it is important to develop an effective vaccine to protect pigs against PEDV infection. The COE epitope of PEDV spike glycoprotein showed neutralizing activities against PEDV (Chang et al. 2002); the COE epitope alone or fusion proteins with mucosal carriers have been expressed in various kinds of plants, including tobacco (Kang et al. 2005, 2006), lettuce (Huy et al. 2009, 2011) and rice endosperm (Oszvald et al. 2007). Rice is one of the most important food crops and the staple food for more than half the world’s population, particularly in Asian countries (Khush 2005). Recently, a transgenic rice grain has been developed for use as a mucosal vaccine, which possesses several advantages (Takagi et al. 2005; Takaiwa 2007; Tokuhara et al. 2010). In the present experiment, prior to development of transgenic rice, the synthetic COE–Co1 fusion protein was produced in transgenic rice calli, and its immunogenicity was tested in mice.
Transgenic plants have been widely used as production system to express heterologous proteins for vaccine antigens. These plants should decrease the required amount of plant-based edible vaccine because it is often painful for animals to consume a large amount of plant material during a short period of time. A lower expression level of antigen protein in transgenic plants means that the feed amount has to be increased, which can be painful and stressful to animals. In addition, oral immunization of a vaccine with low expression level of antigens in transgenic plants induces low immune responses and immune tolerances. The improvement of the expression level of antigen genes in transgenic plants and the delivery of plant-produced antigens into the mucosal immune system could result in enhancement of immune responses. The COE gene, which has been modified based on the plant-optimized codon usage, produces fivefold higher levels of antigens than that expressed with the native gene (Kang et al. 2005). The expression level of recombinant protein under the control of RAmy3D expression system showed a 1,000-fold enhanced expression level in a rice suspension culture system (Shin et al. 2003). In the present study, codon optimization of the target gene and RAmy3D promoter expression system were applied, and the expression level of the COE–Co1 fusion protein was approximately to 0.083 % of TSP in transgenic rice calli at day 3 after induction.
Plant-based oral vaccines elicited immune tolerance and low immune responses against the antigen due to the low expression level of antigen proteins in transgenic plants. The use of ligands to deliver vaccine antigens into mucosal immune systems should be considered to overcome the low immune response in plant-based edible vaccines. The cholera toxin B subunit (CTB) and enterotoxigenic Escherichia coli enterotoxin B subunit (LTB) have been used to deliver fused antigens into mucosal immune systems and have efficiently induced immune responses. However, these ligands have a size limitation of fused antigen proteins for assembling biologically active ligand-fusion proteins and cannot be used repeatedly in mice previously immunized with CTB and LTB fusion proteins. The M cell-target peptide ligand Co1 (with 12 amino acids) has been shown to be an effective delivery method of Co1-fused antigen to mucosal immune inductive sites through M cell targeting without a size limitation of fused proteins and induces the enhanced immune responses against the fused antigen as compared to those of the antigen without a ligand (Kim et al. 2010a).
Although we attempted to improve the expression level of antigen protein by codon optimization of genes for plants using a strong expression system, the expression level was still low relative to the amount fed to the animals. The total soluble proteins were extracted and precipitated using ammonium sulfate to concentrate plant-produced antigens and investigate the immunogenicity of rice-produced COE–Co1 fusion proteins. The precipitation of plant-produced antigen with ammonium sulfate is a non-specific way to increase the amount of proteins and is acceptable for developing animal vaccines (Molina et al. 2005; Martinez et al. 1992). The plant-produced antigens were enriched more than 15-fold, allowing the feeding doses to increase and the feeding volumes to decrease. The rice-produced COE–Co1 fusion protein-induced IgG antibody in immunized mouse sera and secreted secretory IgA (S-IgA) antibody into immunized mouse feces against COE with long-term oral administration. The S-IgA is the predominant antibody class in external secretions and the major humoral defense factor that serves to prevent mucosal surfaces from infective agents, such as bacteria and viruses (de Haan et al. 1995; Woof and Mestecky 2005). This S-IgA may be beneficial in preventing PEDV infection.
The COE-specific IgG and IgA antibody-producing cells in lymphocytes from spleens of mice immunized with plant-derived COE–Co1 fusion protein were detected in more populations compared to that in non-immunized mice, which indicates systemic immune responses. The Peyer’s patches of the lymphocytes isolated from mice immunized with plant-derived COE–Co1 fusion protein showed a twofold larger population of cells secreting anti-COE IgA antibody compared to that in the control mice, indicating that mucosal immune responses were elicited. Therefore, the transgenic rice calli containing COE–Co1 fusion protein induced systemic and mucosal immune responses against the COE antigen for development of a mucosal vaccine against PEDV.
In this study, the sCOE of a PEDV and M cell-target peptide (Co1) fusion gene was constructed and expressed in transgenic rice under the control of an amylase 3D promoter expression system. The mice immunized with concentrated plant protein extracts elicited systemic and mucosal immune responses, suggesting the possibility of a plant-based oral vaccine against PEDV infection.
References
Bae JL, Lee JG, Kang TJ et al (2003) Induction of antigen-specific systemic and mucosal immune responses by feeding animals transgenic plants expressing the antigen. Vaccine 21:4052–4058
Cavanagh D (2005) Coronaviridae: a review of coronaviruses and toroviruses. Birkhäuser Verlag, Basel, pp 1–54
Cavanagh D, Britton P (2008) Coronaviruses: general features. Encyclopedia of virology. Academic Press, New York, pp 549–554
Cavanagh D, Brian D, Brinton MA et al (1993) The Coronaviridae now comprises two genera, coronavirus and torovirus: report of the Coronaviridae Study Group. Adv Exp Med Biol 342:255–257
Chang SH, Bae JL, Kang TJ et al (2002) Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol Cells 14:295–299
Chen L, Marmey P, Taylor NJ et al (1998) Expression and inheritance of multiple transgenes in rice plants. Nat Biotechnol 16:1060–1064
Chu CC, Wang CC, Sun CS et al (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci Sin 18:659–668
Coligan JE (1996) Current protocols in protein science. Wiley, New York, pp A.3F.1–A.3F.8
de Haan A, Renegar KB, Small PA Jr et al (1995) Induction of a secretory IgA response in the murine female urogenital tract by immunization of the lungs with liposome-supplemented viral subunit antigen. Vaccine 13:613–616
Duarte M, Laude H (1994) Sequence of the spike protein of the porcine epidemic diarrhoea virus. J Gen Virol 75(Pt 5):1195–1200
Ducatelle R, Coussement W, Charlier G et al (1981) Three-dimensional sequential study of the intestinal surface in experimental porcine CV 777 coronavirus enteritis. Zentralbl Veterinarmed B 28:483–493
Egberink HF, Ederveen J, Callebaut P et al (1988) Characterization of the structural proteins of porcine epizootic diarrhea virus, strain CV777. Am J Vet Res 49:1320–1324
Follis KE, York J, Nunberg JH (2006) Furin cleavage of the SARS coronavirus spike glycoprotein enhances cell–cell fusion but does not affect virion entry. Virology 350:358–369
Hong SY, Kwon TH, Jang YS et al (2006) Production of bioactive human granulocyte-colony stimulating factor in transgenic rice cell suspension cultures. Protein Expr Purif 47:68–73
Huy NX, Kim YS, Jun SC et al (2009) Production of a heat-labile enterotoxin B subunit-porcine epidemic diarrhea virus-neutralizing epitope fusion protein in transgenic lettuce (Lactuca sativa). Biotechnol Bioprocess Eng 14:731–737
Huy NX, Yang MS, Kim TG (2011) Expression of a cholera toxin B subunit-neutralizing epitope of the porcine epidemic diarrhea virus fusion gene in transgenic lettuce (Lactuca sativa L.). Mol Biotechnol 48:201–209
Hwang YS, Karrer EE, Thomas BR et al (1988) Three cis-elements required for rice alpha-amylase Amy3D expression during sugar starvation. Plant Mol Biol 36:331–341
Jespersgaard C, Hajishengallis G, Greenway TE et al (1999) Functional and immunogenic characterization of two cloned regions of Streptococcus mutans glucosyltransferase I. Infect Immun 67:810–816
Kang TJ, Kang KH, Kim JA et al (2004) High-level expression of the neutralizing epitope of porcine epidemic diarrhea virus by a tobacco mosaic virus-based vector. Protein Expr Purif 38:129–135
Kang TJ, Kim YS, Jang YS et al (2005) Expression of the synthetic neutralizing epitope gene of porcine epidemic diarrhea virus in tobacco plants without nicotine. Vaccine 23:2294–2297
Kang TJ, Han SC, Yang MS et al (2006) Expression of synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat-labile enterotoxin in tobacco plants. Protein Expr Purif 46:16–22
Khush G (2005) What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol 59:1–6
Kim NS, Kim TG, Jang YS et al (2008a) Amylase gene silencing by RNA interference improves recombinant hGM-CSF production in rice suspension culture. Plant Mol Biol 68:369–377
Kim NS, Kim TG, Kim OH et al (2008b) Improvement of recombinant hGM-CSF production by suppression of cysteine proteinase gene expression using RNA interference in a transgenic rice culture. Plant Mol Biol 68:263–275
Kim SH, Seo KW, Kim J et al (2010a) The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol 185:5787–5795
Kim TG, Kim BG, Kim MY et al (2010b) Expression and immunogenicity of enterotoxigenic Escherichia coli heat-labile toxin B subunit in transgenic rice callus. Mol Biotechnol 44:14–21
Kim MY, Yang MS, Kim TG (2012) Expression of a consensus dengue virus envelope protein domain III in transgenic callus of rice. Plant Cell Tissue Organ Cult 109:509–515
Kocherhans R, Bridgen A, Ackermann M et al (2001) Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 23:137–144
Kong Q, Richter L, Yang YF et al (2001) Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci USA 98:11539–11544
Lee HK, Yeo SG (2003) Biological and physicochemical properties of porcine epidemic diarrhea virus Chinju99 strain isolated in Korea. J Vet Clin 20:150–154
Lehrach H, Diamond D, Wozney JM et al (1977) RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 16:4743–4751
Martinez C, Dalsgaard K, Lopez de Turiso JA et al (1992) Production of porcine parvovirus empty capsids with high immunogenic activity. Vaccine 10:684–690
Molina A, Veramendi J, Hervas-Stubbs S (2005) Induction of neutralizing antibodies by a tobacco chloroplast-derived vaccine based on a B cell epitope from canine parvovirus. Virology 342:266–275
Oszvald M, Kang TJ, Tomoskozi S et al (2007) Expression of a synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat labile enterotoxin in rice endosperm. Mol Biotechnol 35:215–223
Shin YJ, Hong SY, Kwon TH et al (2003) High level of expression of recombinant human granulocyte–macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol Bioeng 82:778–783
Takagi H, Hiroi T, Yang L et al (2005) A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc Natl Acad Sci USA 102:17525–17530
Takaiwa F (2007) A rice-based edible vaccine expressing multiple T-cell epitopes to induce oral tolerance and inhibit allergy. Immunol Allergy Clin North Am 27:129–139
Tokuhara D, Yuki Y, Nochi T et al (2010) Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc Natl Acad Sci USA 107:8794–8799
Woof JM, Mestecky J (2005) Mucosal immunoglobulins. Immunol Rev 206:64–82
Yeo SG, Hernandez M, Krell P et al (2003) Cloning and sequence analysis of the spike gene of porcine epidemic diarrhea virus Chinju99. Virus Genes 26:239–246
Yoshida T, Kimura E, Koike S et al (2011) Transgenic rice expressing amyloid beta-peptide for oral immunization. Int J Biol Sci 7:301–307
Acknowledgments
This study was supported by the Technology Development Program for Agriculture and Forestry, Ministry for Agriculture, Forestry and Fisheries, Republic of Korea and by research funds of Chonbuk National University in 2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Toriyama.
Rights and permissions
About this article
Cite this article
Huy, NX., Kim, SH., Yang, MS. et al. Immunogenicity of a neutralizing epitope from porcine epidemic diarrhea virus: M cell targeting ligand fusion protein expressed in transgenic rice calli. Plant Cell Rep 31, 1933–1942 (2012). https://doi.org/10.1007/s00299-012-1306-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1306-0