Abstract
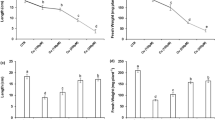
In this study, we examined the modulation of Cu toxicity-induced oxidative stress by excess supply of iron in Zea mays L. plants. Plants receiving excess of Cu (100 μM) showed decreased water potential and simultaneously showed wilting in the leaves. Later, the young leaves exhibited chlorosis and necrotic scorching of lamina. Excess of Cu suppressed growth, decreased concentration of chloroplastic pigments and fresh and dry weight of plants. The activities of peroxidase (EC 1.11.1.7; POD), ascorbate peroxidase (EC 1.11.1.11; APX) and superoxide dismutase (EC 1.15.1.1; SOD) were increased in plants supplied excess of Cu. However, activity of catalase (EC 1.11.1.6; CAT), was depressed in these plants. In gel activities of isoforms of POD, APX and SOD also revealed upregulation of these enzymes. Excess (500 μM)-Fe-supplemented Cu-stressed plants, however, looked better in their phenotypic appearance, had increased concentration of chloroplastic pigments, dry weight, and improved leaf tissue water status in comparison to the plants supplied excess of Cu. Moreover, activities of antioxidant enzymes including CAT were further enhanced and thiobarbituric acid reactive substance (TBARS) and H2O2 concentrations decreased in excess-Fe-supplemented Cu-stressed plants. In situ accumulation of H2O2, contrary to that of O2 ·− radical, increased in both leaf and roots of excess-Cu-stressed plants, but Cu-excess plants supplied with excess-Fe showed reduced accumulation H2O2 and little higher of O2 ·− in comparison to excess-Cu plants. It is, therefore, concluded that excess-Cu (100 μM) induces oxidative stress by increasing production of H2O2 despite of increased antioxidant protection and that the excess-Cu-induced oxidative damage is minimized by excess supply of Fe.








Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CAT:
-
Catalase
- DAT:
-
Days after treatments
- EDTA:
-
Ethylenediamine tetraacetic acid
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
References
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Anderson MD, Prasad TK, Stewart CR (1995) Changes in isozyme profile of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109:1247–1257
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–379
Babu TS, Akhtar TA, Lampi MA, Tripuranthakam S, Dixon DG, Greenberg BM (2003) Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemna gibba: implication of reactive oxygen species as common signals. Plant Cell Physiol 44:1320–1329
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and assays applicable to acrylamide gels. Anal Biochem 44:276–287
Becana M, Moran JF, Iturbe-Ormaetxe I (1998) Iron dependent oxygen free radical generation in plants subjected to environmental stress: toxicity and antioxidant protection, Plant Soil 201:137–147
Bisht SS, Sharma A, Chaturvedi K (1989) Certain metabolic lesions of chromium toxicity in radish. Indian J Agric Biochem 2:109–115
Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in regulation of senescence in pear. Plant Physiol 59:411–416
Burzyński M, Klobus G (2004) Changes in photosynthetic parameters in cucumber leaves under Cu, Cd, and Pb stress. Photosynthetica 142:505–510
Chen Y, Shi J, Tian G, Zheng S, Lin Qi (2004) Fe deficiency induces Cu uptake and accumulation in Commelina communis. Plant Sci 166:1371–1377
Cornu JY, Staunton S, Hinsinger P (2007) Copper concentration in plants and in the rhizosphere as influenced by the iron status of tomato (Lycopersicon esculentum L.). Plant Soil 292:63–77
Doncheva S, Stoyanova Z, Georgieva K, Nedeva D, Dikova R, Zehirov G, Nikolova A (2006) Exogenous succinate increases resistance of maize plants to copper stress. J Plant Nutr Soil Sci 169:247–254
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Hames BD (1990) One dimensional polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D (eds) Gel electrophoresis of protein. Oxford University Press, England, pp 1–87
Hauck M, Paul A Gross S, Raubuch M (2003) Manganese toxicity in epiphytic lichens: chlorophyll degradation and interaction with iron and phosphorus. Environ Exp Bot 49:181–191
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast, I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:180–198
Hernández JA, Ferrer MA, Jiménez A, Barceló AR, Sevilla F (2001) Antioxidant systems and O2 ·−/H2O2 production in the apoplast of pea leaves, its relation with salt–induced necrotic lesions in minor veins. Plant Physiol 127:817–831
Hewitt EJ (1963) The essential nutrient elements: requirements and interactions in plants. In: Steward FC (ed) Plant physiology, vol III. Academic, New York, pp 137–360
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. England: commonwealth agricultural bureaux, Farnham Royl. Bucks
Huang Y, Tao S (2004) Influences of excessive Cu on photosynthesis and growth in ectomycorrhizal Pinus sylvestris seedlings. J Environ Sci (China) 16:414–419
Iturbe-Ormaetxe I, Moran JF, Arrese-Igor C, Gogorcena Y, Klucas RV, Becana M (1995) Activated oxygen and antioxidant defences in iron-deficient pea plants. Plant Cell Environ 18:421–429
Kampfenkel K, Montagu MV, Inzé D (1995) Effect of iron excess on Nicotiana plumbaginifolia plants: implication to oxidative stress. Plant Physiol 107:725–735
Lombardi L, Sebastiani L (2005) Copper toxicity in Prunus cerasifera: growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci 168:797–802
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Luck H (1963) Peroxidase. In: Bergmeyer HU (ed) Methods of enzymic analysis, Academic, New York, pp 895–897
Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34:321–342
Maksymiec W, Krupa Z (2006) The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ Exp Bot 57:187–194
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Mehrotra SC, Mehrotra NK, Bisht SS, Sharma CP (1976) Resolution of iron chlorosis. Goephytology 6:282–295
Mittler R, Zilinskas BA (1993) Detection of ascorbate peroxidase activity in native gels by inhibition of ascorbate-dependent reduction of nitroblue tetrazolium. Anal Biochem 212:540–546
Morelli E, Scarano G (2004) Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalga Phaeodactylum tricornutum. Plant Sci 167:289–296
Motta A, Basso B, Dell’Orto M, Briat JF, Soave C (2001) Ferritin synthesis in response to iron in the Fe-inefficient maize mutant ys3. Plant Physiol Biochem 39:461–465
Murao K, Takamiya M, Ono K, Takano H, Takio S (2004) Copper deficiency induced expression of Fe-superoxide dismutase gene in Matteuccia struthiopteris. Plant Physiol Biochem 42:143–148
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Pätsikkä E, Aro EM, Tyystjärvi E (2001) Mechanism of copper-enhanced photoinhibition in thylakoid membranes. Physiol Plant 113:142–150
Pätsikkä E, Kairavuo M, Šeršen F, Aro EM, Tyystjärvi E (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367
Perales-Vela HV, González-Moreno S, Montes-Horeasitas C, Cañizares-Villanueva RO (2007) Growth, photosynthetic and respiratory responses to sub-lethal copper concentrations in Scenedesmus incrassatulus (Chlorophyceae). Chemosphere 67:2274–2281
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipid and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Exp Bot 52:77–84
Raeymaekers T, Potters G, Asard H, Guisez Y, Horemans N (2003) Copper-mediated oxidative burst in Nicotiana tabacum L. cv. Bright Yellow 2 cell suspension cultures. Protoplasma 221:93–100
Reuther W, Labanauskas CK (1966) Copper. In: Chapman HD (ed) Diagnostic criteria for plants and soils. Division of agricultural sciences, University of California, California, pp 157–179
Schmidt W, Bartels M, Tittel J, Fühner C (1997) Physiological effects on iron acquisition processes in Plantago. New Phytol 135:659–666
Shingles R, Wimmers LE, McCarty RE (2004) Copper transport across pea thylakoid membranes. Plant Physiol 135:145–151
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Taylor GJ, Foy CD (1985) Differential uptake and toxicity of ionic and chelated copper in Triticum aestivum. Can J Bot 63:1271–1275
Tewari RK, Kumar P, Sharma PN (2006) Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta 223:1145–1153
Tewari RK, Hahn E-J, Paek K-Y (2007) Modulation of copper toxicity-induced oxidative damage by nitric oxide supply in the adventitious roots of Panax ginseng. Plant Cell Rep. doi:10.1007/s00299-007-0423-7
Vassilev A, Lidon F, Scotti Campos P, Ramalho JC, Barreiro MG, Yordanov (2003) Cu induced changes in chloroplast lipids and photosystem 2 activity in barley leaves. Bulg J Plant Physiol 29:33–43
Ylivainio K, Jaakkola A, Aksela R (2004) Effects of Fe compounds on nutrient uptake by plants grown in sand media with different pH. J Plant Nutr Soil Sci 167:602–608
Acknowledgments
Authors (P. K. and R. K. T.) are grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial supports. Dr. P.K. Misra, Dr. Neetu and Dr. Shyam Kishore are gratefully acknowledged for their help in microphotography and Dr. Amit Gupta for his help in the measurement of metals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Feher.
Rights and permissions
About this article
Cite this article
Kumar, P., Tewari, R.K. & Sharma, P.N. Modulation of copper toxicity-induced oxidative damage by excess supply of iron in maize plants. Plant Cell Rep 27, 399–409 (2008). https://doi.org/10.1007/s00299-007-0453-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0453-1