Abstract
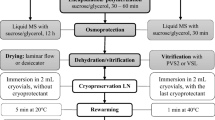
Shoot tips of sweet potato were successfully cryopreserved using an encapsulation vitrification method. Encapsulated shoot tips were pre-incubated in liquid Murashige-Skoog medium containing 30 g/l sucrose for 24 h, then precultured in sucrose-enriched medium (0.3 M sucrose) for 16 h. Shoot tips were osmoprotected with a mixture of 2 M glycerol and 1.6 M sucrose for 3 h before being dehydrated with a highly concentrated vitrification solution (PVS2) for 1 h at 25°C. The encapsulated and dehydrated shoot tips were transferred to a 2 ml cryotube, suspended in 0.5 ml PVS2, and plunged directly into liquid nitrogen. Rapidly warmed shoot tips developed normal shoots and roots in 21 days without any morphological abnormalities after plating on a recovery medium. High levels (average of about 80%) of shoot formation were obtained for three cultivars of sweet potato. This encapsulation vitrification method appears promising for cryopreservation of sweet potato germplasm.






Similar content being viewed by others
Abbreviations
- BA :
-
6-Benzyl aminopurine
- DMSO :
-
Dimethyl sulfoxide
- GA 3 :
-
Gibberellic acid 3
- IAA :
-
Indole-3-acetic acid
- LN :
-
Liquid nitrogen
- MS :
-
Murashige-Skoog medium
- NAA :
-
1-Naphthaleneacetic acid
References
Bajaj YPS (1978) Tuberization in potato plants regenerated from freeze-preserved meristems. Crop Improv 5:137–141
Charoensub R, Phansiri S, Sakai A, Yongmanitchai W (1999) Cryopreservation of cassava in vitro-grown shoot tips cooled to −196°C by vitrification. Cryo-Lett 20:89–94
Fabre J, Dereuddre J (1990) Encapsulation-dehydration: a new approach to cryopreservation of Solanum shoot-tips. Cryo-Lett 11:413–426
Fukai S, Goi M, Tanaka M (1991) Cryopreservation of shoot tips of Chrysanthemum morifolium and related species native to Japan. Euphytica 54:201–204
Harding K, Benson EE (1994) A study of growth, flowering and tuberization in plants derived from cryopreserved shoot-tips of Solanum tuberosum. Cryo-Lett 15:59–66
Hellergren J, Li PH (1981) Survival of Solanum tuberosum suspension cultures to −14°C: the mode of action of proline. Physiol Plant 52:449–453
Hirai D (2001) Studies on cryopreservation of vegetatively propagated crops by encapsulation vitrification method. Rep Hokkaido Pref Agric Exp Stn 99:1–58
Hirai D, Sakai A (1999a) Cryopreservation of in vitro-grown axially shoot tip meristems of mint (Mentha spicata L.) by encapsulation vitrification. Plant Cell Rep 19:150–155
Hirai D, Sakai A (1999b) Cryopreservation of in vitro-grown meristems of potato (Solanum tuberosum L.) by encapsulation-vitrification. Potato Res 42:153–160
Hirai D, Sakai A (2001) Recovery growth of plants cryopreserved by encapsulation-vitrification. Bull Hokkaido Agric Exp Stn 80:55–64
Hirai D, Shirai K, Shirai S, Sakai A (1998) Cryopreservation of in vitro-grown meristems of strawberry (Fragaria × ananassa Duch.) by encapsulation-vitrification. Euphytica 101:109–115
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (1997) Ultrastructural study of mechanism of increased freezing tolerance to extracellular glucose in cabbage leaf cells. Cryo-Lett 18:33–44
Kyesmu PM, Takagi H (2000) Cryopreservation of shoot apices of yams (Dioscorea species) by vitrification. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. JIRCAS, Tsukuba, Japan, pp 411–413
Lambardi M, Fabbri A, Caccavale A (2000) Cryopreservation of white poplar (Populus alba L.) by vitrification of in vitro-grown shoot tips. Plant Cell Rep 19:213–218
Langis R, Schnabel-Preikstas BJ, Earle ED, Steponkus PL (1990) Cryopreservation of carnation shoot tips by vitrification. Cryobiology 27:657–658
Matsumoto T, Sakai S, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Matsumoto T, Sakai A, Takahashi C, Yamada K (1995a) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabi japonica) by encapsulation-vitrification method. Cryo-Lett 16:189–206
Matsumoto T, Sakai A, Yamada K (1995b) Cryopreservation of in vitro-grown apical meristems of lily by vitrification. Plant Cell Tissue Organ Cult 41:237–241
Matsumoto T, Sakai A, Nako Y (1998) A novel preculturing for enhancing the survival of in vitro-grown meristems of wasabi (Wasabia japonica) cooled to −196°C by vitrification. Cryo-Lett 19:27–36
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Niino T, Seguel I, Murayama T (2000) Cryopreservation of vegetatively propagated species (mainly mulberry). In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. JIRCAS, Tsukuba, Japan, pp 194–199
Pennycooke JC, Towill LE (2000) Cryopreservation of shoot tips from in vitro plants of sweet potato [Ipomoea batatas (L.) Lam.] by vitrification. Plant Cell Rep 19:733–737
Pennycooke JC, Towill LE (2001) Medium alternations improve regrowth of sweet potato (Ipomoea batatas [L.] Lam.) shoot tips cryopreserved by vitrification and encapsulation-dehydration. Cryo-Lett 22:381–389
Reinhoud PJ (1996) Cryopreservation of tobacco suspension cells by vitrification. Doctoral Paper, Rijks University, Leiden, pp 1–95
Sakai A (1995) Cryopreservation of germplasm of woody plants. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 52. Springer, Berlin Heidelberg, New York, pp 53–69
Sakai A (2000) Development of cryopreservation techniques. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. JIRCAS, Tsukuba, Japan, pp 1–7
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sakai A, Matsumoto T, Hirai D, Charoensub R (2002) Survival of tropical apices cooled to −196°C by vitrification—development of a potential cryogenic protocol of tropical plants by vitrification. In: Li PH, Palva ET (eds) Plant cold hardiness—gene regulation and genetic engineering. Kluwer/Plenum, New York, pp 109–119
Takagi H, Thinh NT, Islam OM, Senboku T, Sakai A (1997) Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedures. Plant Cell Rep 16:594–599
Takagi H, Thinh NT, Kyesm OM (1998) Cryopreservation of vegetatively propagated tropical crops by vitrification. Acta Hortic 461:485–495
Tao D, Li PH, Carter JV (1983) Role of cell wall in freezing tolerance of cultured potato cells and their protoplasts. Physiol Plant 58:527–532
Thinh NT, Takagi H (2000) Cryopreservation of in vitro-grown apical meristems of terrestrial orchids (Cymbidium spp) by vitrification. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. JIRCAS, Tsukuba, Japan, pp 441–443
Thinh NT, Takagi H, Yashima S (1999) Cryopreservation of in vitro-grown shoot tips of banana (Musa spp) by vitrification method. Cryo-Lett 20:163–174
Touchell DH, Dixon KW (1995) Cryopreservation for the conservation of native Australian endangered plants. In: Normah MN, Narimah MK, Clyde MM (eds) In-vitro conservation of plant genetic resources. University Kebangsaan, Malaysia, pp 169–180
Yamada T, Sakai A, Matsumura T, Higuchi S (1991) Cryopreservation of apical meristems of white clover (Trifolium repens L.) by vitrification. Plant Sci 78:81–87
Acknowledgement
The authors wish to thank Dr. Kei Shimonishi, Kagoshima Biotechnology Institute for supplying in-vitro-grown sweet potato plants
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.C. Phillips
Rights and permissions
About this article
Cite this article
Hirai, D., Sakai, A. Simplified cryopreservation of sweet potato [Ipomoea batatas (L.) Lam.] by optimizing conditions for osmoprotection. Plant Cell Rep 21, 961–966 (2003). https://doi.org/10.1007/s00299-003-0618-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0618-5