Abstract
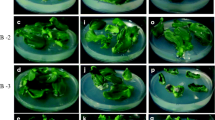
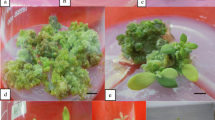
Seedling-derived cotyledon explants of squash (Cucurbita pepo L.) of commercial cultivars True French, Ma'yan and Goldy were regenerated in vitro on Murashige and Skoog medium augmented with 1 mg/l benzyladenine. After 4 weeks in culture small shoots and buds regenerated only on the most proximal cotyledon edge. Culture on an elongation medium with a reduced cytokinin concentration (0.1 mg/l) with or without 1 mg/l gibberellic acid (GA3) facilitated the recovery of shoots. Fresh shoots could be recovered at each subculture of the regenerating mass. Peak productivity was during the third cycle of subculture, and shoot production ceased after the fifth subculture. Culture on elongation medium supplemented with GA3 was 55% more effective with respect to overall shoot production than that on medium without GA3, with 22 shoots recovered in total per explant from the former. Regeneration occurred under both light and dark conditions. All of the shoots tested were diploid. The shoots were rooted and transferred to the greenhouse where they grew and flowered normally.




Similar content being viewed by others
Abbreviations
- BA :
-
Benzyladenine
- GA 3 :
-
Gibberellic acid
- SEM :
-
Scanning electron microscopy
References
Chee PP (1991) Somatic embryogenesis and plant regeneration of squash Cucurbita pepo L. cv. YC 60. Plant Cell Rep 9:620–622
Chee PP (1992) Initiation and maturation of somatic embryos of squash (Cucurbita pepo). HortScience 27:59–60
Colijn-Hooymans CM, Hakkert JC, Jansen J, Custers JMB (1994) Competence for regeneration of cucumber cotyledons is restricted to specific developmental stages. Plant Cell Tissue Organ Cult 39:211–217
Compton ME (1999) Dark pretreatment improves adventitious shoot organogenesis from cotyledons of diploid watermelon. Plant Cell Tissue Organ Cult 58:185–188
Compton ME, Gray DJ, Elmstrom GW (1996) Identification of tetraploid regenerants from cotyledons of diploid watermelon cultured in vitro. Euphytica 87:165–172
Curuk S, Elman C, Schlarman E, Sagee O, Shomer I, Cetiner S, Gray DJ, Gaba V (2002) A novel pathway for rapid shoot regeneration from the proximal zone of the hypocotyl of melon (Cucumis melo L.). In Vitro Cell Dev Biol Plant 38:260–267
Curuk S, Ananthakrishnan G, Singer S, Xia X, Elman C,Nestel D,Cetiner S, Gaba V (2003) Regeneration in vitro from the hypocotyl of Cucumis species produces almost exclusively diploid shoots, and does not require light. HortScience (in press)
De Laat AMM, Blaas J (1984) Flow cytometric characterization and sorting of plant chromosomes. Theor Appl Genet 67:463–467
Debeaujon I, Branchard M (1993) Somatic embryogenesis in Cucurbitaceae. Plant Cell Tissue Organ Cult 34:91–100
Dong JZ, Jia SR (1991) High efficiency plant regeneration from cotyledons of watermelon (Citrullus vulgaris Schrad.). Plant Cell Rep 9:559–562
Ezura H, Amagi H, Yoshioka K, Oosawa K (1992) Highly frequent appearance of tetraploidy in regenerated plants, a universal phenomenon in tissue-cultured melon (Cucumis melo L.) Plant Sci 85:209–213
FAO (1999) FAO production handbook 53:141–142
Gaba V, Elman E, Watad AA, Gray DJ (1996) Ancymidol hastens in vitro bud development in melon. HortScience 31:1223–1224
Gambley RL, Dodd WA (1991) The influence of cotyledons in axillary and adventitious shoots production from cotyledonary nodes of Cucumis sativus L. J Exp Bot 42:1131–1135
George EF (1993) Plant propagation by tissue culture, part 1, 2nd edn. The technology. Exegetics, Edington
Girija S, Ganapathi A, Vengadesan G (1999) Micropropagaation of Crossandra infundibuliformis (L.) Nees. Sci Hortic 82:331–337
Gonzalves C, Xue B, Gonsalves D (1995) Somatic embryogenesis and regeneration from cotyledon explants of six squash cultivars. HortScience 30:1295–1297
Jelaska S (1972) Embryoid formation by fragments of cotyledons and hypocotyls in Cucurbita pepo. Planta 103:278–280
Jelaska S (1974) Embryogenesis and organogenesis in pumpkin explants. Physiol Plant 31:257–261
Jelaska S (1986) Cucurbits. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 2. Crops I. Springer, Berlin Heidelberg New York, pp 371–386
Lercari B, Tognoni F, Anselmo G, Chapel D (1986) Photocontrol of in vitro bud differentiation in Saintpaulia ionantha leaves and Lycopersicon esculentum cotyledons. Physiol Plant 67:340–344
Malone-Schoneberg J, Scelong CJ, Burrus M, Bidney DL (1994) Stable transformation of sunflower using Agrobacterium and split embryogenic axis explants. Plant Sci 103:199–207
Mante S, Scorza R, Cordts J (1989) A simple, rapid protocol for adventitious shoot development from mature cotyledons of Glycine max cv. Bragg. In Vitro Cell Dev Biol 25:385–388
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–498
Neidz RP, Smith SS, Dunbar KV, Stephens CT, Murakishi HH (1989) Factors affecting shoot regeneration from cotyledonary explants of Cucumis melo. Plant Cell Tissue Organ Cult 18:313–319
Paris HS (1989) Historical records, origins, and development of the edible cultivar groups of Cucurbita pepo (Cucurbitaceae). Econ Bot 43:423–443
Paris HS, Cohen S (2000) Oligogenic inheritance for resistance to Zucchini Yellow Mosaic Virus in Cucurbita pepo. Ann Appl Biol 136:209–214
Rahman SM, Hossain M, Islam R, Joarder OI (1993) Plant regeneration from internode segments of Cucurbita maxima Duch.×Cucurbita moschata Duch. Curr Sci 65:562–564
Rakoczy-Trojanowska M, Malepszy S (1989) A method for increased plant regeneration from immature F1 and BC1 embryos of Cucubita maxima Duch.×C. pepo L. hybrids. Plant Cell Tissue Organ Cult 18:191–194
Ravikumar R, Ananthakrishnan G, Kathiravan K, Ganapathi A (1998) In vitro shoot propagation of Dendrocalamus strictus Nees. Plant Cell Tissue Organ Cult 52:189–192
Rubos AC, Pryke AA (1984) Morphogenesis in embryogenic tissue cultures of apple. J Hortic Sci 59:469–475
Sharma KK, Bhojwani SS (1990) Histological aspects of in vitro root and shoot differentiation from cotyledon explants of Brassica juncea (L.) Czern. Plant Sci 69:207–214
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. W.H. Freeman, New York
Tricoli DM, Carney KJ, Russel PF, McMaster JR, Groff DW, Hadden KC, Himmel PT, Hubbard JP, Boeshore ML, Reynolds JF, Quemada HD (1995) Field evaluation of transgenic squash containing single or multiple virus coat protein gene constructs for resistance to cucumber mosaic virus, watermelon mosaic virus 2, and/or zucchini yellow mosaic virus. Biotechnology 13:1458–1465
Wang S, Tang L, Chen F (2001) In vitro flowering of bitter melon. Plant Cell Rep 20:393–397
Acknowledgements
This investigation was supported by Research Grant no. US2541-95R from BARD, The United States-Israel Binational Agricultural Research and Development Fund to V.G. and A.G. G.A. was supported by the MASHAV program of the Ministry of Foreign Affairs, Israel. The authors thank the late Dr. Erwin Fischer for assistance with the scanning electron microscopy, and Drs. B. Steinitz and A. Zelcer for critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.C. Phillips
Contribution from the Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel, no. 510/02.
Rights and permissions
About this article
Cite this article
Ananthakrishnan, G., Xia, X., Elman, C. et al. Shoot production in squash (Cucurbita pepo) by in vitro organogenesis. Plant Cell Rep 21, 739–746 (2003). https://doi.org/10.1007/s00299-003-0584-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0584-y