Abstract
Behçet’s syndrome (BS) is a chronic (auto)-inflammatory disorder characterized by different clusters of symptoms, including mucocutaneous and ocular involvements. Interleukin-1 inhibitors anakinra (ANA), canakinumab (CAN), and gevokizumab (GEV) represent a promising therapeutic alternative in BS. To date, evidence on the use of ANA, CAN, and GEV is mainly based on small isolated studies or case series, and the real place of anti-IL1 agents in the treatment of BS is still unclear. We performed a systematic review of current evidence on the efficacy and safety of anti-IL1 agents in BS. The PubMed search yielded a total of 398 references, from which we retrieved 24 studies for inclusion (4 clinical trials, 6 observational studies, 14 case reports, case series or letters to the editor). Four studies evaluated the overall efficacy of IL-1 inhibitors, 15 studies focused on the specific efficacy of ANA, whereas efficacy of CAN and GEV was evaluated in 8 and 3 studies, respectively. Both ANA and CAN were associated with good control of mucocutaneous and ocular manifestations. ANA resulted effective also for osteoarticular manifestations. GEV was studied only for ocular manifestations, but gave contrasting results. Discordant evidence supports the use of ANA and CAN in pediatric setting and for first-line treatment of general BS manifestations. Most frequent side effects were local or diffuse cutaneous reactions and injection site reactions, particularly for ANA treatment. Blocking the IL-1 pathway could be an effective therapeutic strategy in particular BS involvements.


Similar content being viewed by others
References
Emmi G, Silvestri E, Squatrito D et al (2014) Behçet’s syndrome pathophysiology and potential therapeutic targets. Intern Emerg Med 9:257–265. https://doi.org/10.1007/s11739-013-1036-5
Morton LT, Situnayake D, Wallace GR (2016) Genetics of Behçetʼs disease. Curr Opin Rheumatol 28:39–44. https://doi.org/10.1097/BOR.0000000000000234
Consolandi C, Turroni S, Emmi G et al (2015) Behçet’s syndrome patients exhibit specific microbiome signature. Autoimmun Rev 14:269–276. https://doi.org/10.1016/j.autrev.2014.11.009
Volle G, Fraison J-B, Gobert D et al (2017) Dietary and nondietary triggers of oral ulcer recurrences in Behçet’s disease. Arthritis Care Res (Hoboken) 69:1429–1436. https://doi.org/10.1002/acr.23155
Becatti M, Emmi G, Silvestri E et al (2016) Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behçet disease. Circulation 133:302–311. https://doi.org/10.1161/CIRCULATIONAHA.115.017738
Emmi G, Silvestri E, Bella C, Della et al (2016) Cytotoxic Th1 and Th17 cells infiltrate the intestinal mucosa of Behcet patients and exhibit high levels of TNF-α in early phases of the disease. Medicine (Baltimore) 95:e5516. https://doi.org/10.1097/MD.0000000000005516
Aldinucci A, Bonechi E, Biagioli T et al (2018) CSF/serum matrix metallopeptidase-9 ratio discriminates neuro Behçet from multiple sclerosis. Ann Clin Transl Neurol 5:493–498. https://doi.org/10.1002/acn3.538
Akkoç N (2018) Update on the epidemiology, risk factors and disease outcomes of Behçet’s disease. Best Pract Res Clin Rheumatol 32:261–270. https://doi.org/10.1016/j.berh.2018.08.010
Nanke Y, Yago T, Kotake S (2017) The role of Th17 cells in the pathogenesis of Behcet’s disease. J Clin Med 6:74. https://doi.org/10.3390/jcm6070074
Kirino Y, Nakajima H (2019) Clinical and genetic aspects of Behçet’s disease in Japan. Intern Med. https://doi.org/10.2169/internalmedicine.2035-18
Hatemi G, Seyahi E, Fresko I et al Behçet’s syndrome: a critical digest of the 2013–2014 literature. Clin Exp Rheumatol 32:S112-22
Hatemi G, Christensen R, Bang D et al (2018) 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis 77:808–818. https://doi.org/10.1136/annrheumdis-2018-213225
Emmi G, Vitale A, Silvestri E et al (2018) Adalimumab-based treatment versus DMARDs for venous thrombosis in Behçet syndrome. A retrospective study of 70 patients with vascular involvement. Arthritis Rheumatol. https://doi.org/10.1002/art.40531
Lopalco G, Emmi G, Gentileschi S et al (2017) Certolizumab Pegol treatment in Behcet’s disease with different organ involvement: A multicenter retrospective observational study. Mod Rheumatol 27:1031–1035. https://doi.org/10.1080/14397595.2017.1285857
Urruticoechea-Arana A, Cobo-Ibáñez T, Villaverde-García V et al (2019) Efficacy and safety of biological therapy compared to synthetic immunomodulatory drugs or placebo in the treatment of Behçet’s disease associated uveitis: a systematic review. Rheumatol Int 39:47–58. https://doi.org/10.1007/s00296-018-4193-z
Vitale A, Emmi G, Lopalco G et al (2017) Adalimumab effectiveness in Behçet’s disease: short and long-term data from a multicenter retrospective observational study. Clin Rheumatol 36:451–455. https://doi.org/10.1007/s10067-016-3417-4
Fabiani C, Vitale A, Emmi G et al (2017) Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol 36:183–189. https://doi.org/10.1007/s10067-016-3480-x
Vitale A, Emmi G, Lopalco G et al (2017) Long-term efficacy and safety of golimumab in the treatment of multirefractory Behçet’s disease. Clin Rheumatol 36:2063–2069. https://doi.org/10.1007/s10067-017-3627-4
Vitale A, Rigante D, Lopalco G et al (2016) New therapeutic solutions for Behçet’s syndrome. Expert Opin Investig Drugs 25:827–840. https://doi.org/10.1080/13543784.2016.1181751
Nguyen QD, Merrill PT, Jaffe GJ et al (2016) Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 388:1183–1192. https://doi.org/10.1016/S0140-6736(16)31339-3
Jaffe GJ, Dick AD, Brézin AP et al (2016) Adalimumab in patients with active noninfectious uveitis. N Engl J Med 375:932–943. https://doi.org/10.1056/NEJMoa1509852
Zeydan B, Uygunoglu U, Saip S et al (2016) Infliximab is a plausible alternative for neurologic complications of Behçet disease. Neurol Neuroimmunol neuroinflammation 3:e258. https://doi.org/10.1212/NXI.0000000000000258
Fabiani C, Sota J, Vitale A et al (2017) Ten-Year Retention rate of infliximab in patients with Behçet’s disease-related uveitis. Ocul Immunol Inflamm 1–6. https://doi.org/10.1080/09273948.2017.1391297
Emmi G, Silvestri E, Squatrito D et al (2016) Tocilizumab-induced exacerbation of mucosal ulcers in a patient with multi-refractory Behçet׳s disease. Semin Arthritis Rheum 46:e1–e2. https://doi.org/10.1016/j.semarthrit.2016.03.006
Addimanda O, Pipitone N, Pazzola G, Salvarani C (2015) Tocilizumab for severe refractory neuro-Behçet: three cases IL-6 blockade in neuro-Behçet. Semin Arthritis Rheum 44:472–475. https://doi.org/10.1016/j.semarthrit.2014.08.004
Mirouse A, Barete S, Monfort JB et al (2017) Ustekinumab for Behçet’s disease. J Autoimmun. https://doi.org/10.1016/j.jaut.2017.05.002
Di Scala G, Bettiol A, Cojan RD et al (2018) Efficacy of the anti-IL 17 secukinumab in refractory Behçet’s syndrome: a preliminary study. J Autoimmun. https://doi.org/10.1016/j.jaut.2018.09.002
YIlmaz I, Türk M, Nazik Bahçecioğlu S (2018) Successful rapid subcutaneous desensitization to anakinra in a case with a severe immediate-type hypersensitivity reaction. Eur Ann Allergy Clin Immunol 50:94–96. https://doi.org/10.23822/EurAnnACI.1764-1489.30
Emmi G, Urban ML, Imazio M et al (2018) Use of interleukin-1 blockers in pericardial and cardiovascular diseases. Curr Cardiol Rep 20:61. https://doi.org/10.1007/s11886-018-1007-6
Brucato A, Emmi G, Cantarini L et al (2018) Management of idiopathic recurrent pericarditis in adults and in children: a role for IL-1 receptor antagonism. Intern Emerg Med 13:475–489. https://doi.org/10.1007/s11739-018-1842-x
Colafrancesco S, Priori R, Valesini G et al (2017) Response to interleukin-1 inhibitors in 140 Italian patients with adult-onset still’s disease: a multicentre retrospective observational study. Front Pharmacol 8:369. https://doi.org/10.3389/fphar.2017.00369
Cavalli G, Dinarello CA (2018) Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol 9:1157. https://doi.org/10.3389/fphar.2018.01157
Astrakhantseva IV, Efimov GA, Drutskaya MS et al (2014) Modern anti-cytokine therapy of autoimmune diseases. Biochemistry 79:1308–1321. https://doi.org/10.1134/S0006297914120049
Owyang AM, Issafras H, Corbin J et al XOMA 052, a potent, high-affinity monoclonal antibody for the treatment of IL-1β-mediated diseases. MAbs 3:49–60
Higgins JPT, Altman DG, Gotzsche PC et al (2011) The cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.d5928
Wells GA, Shea B, O’Connell D et al (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst doi. https://doi.org/10.2307/632432
Fabiani C, Vitale A, Rigante D et al (2018) The presence of uveitis is associated with a sustained response to the interleukin (IL)-1 inhibitors anakinra and canakinumab in Behçet’s disease. Ocul Immunol Inflamm 1–7. https://doi.org/10.1080/09273948.2018.1511810
Sota J, Vitale A, Insalaco A et al (2018) Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clin Rheumatol. https://doi.org/10.1007/s10067-018-4119-x
Pagnini I, Bondi T, Simonini G et al (2015) Successful treatment with canakinumab of a paediatric patient with resistant Behçet’s disease. Rheumatology (Oxford) 54:1327–1328. https://doi.org/10.1093/rheumatology/kev197
Emmi G, Silvestri E, Ciucciarelli L et al (2014) Reply: anti-IL1 blocking agents in drug-resistant Behçet’s syndrome: our little case series. Clin Exp Rheumatol 32:S172
Caso F, Rigante D, Vitale A et al (2014) Efficacy of anakinra in refractory Behçet’s disease sacroiliitis. Clin Exp Rheumatol 32:S171
Vitale A, Rigante D, Caso F et al (2014) Inhibition of interleukin-1 by canakinumab as a successful mono-drug strategy for the treatment of refractory behçet’s disease: a case series. Dermatology 228:211–214. https://doi.org/10.1159/000358125
Cantarini L, Vitale A, Scalini P et al (2015) Anakinra treatment in drug-resistant Behcet’s disease: a case series. Clin Rheumatol 34:1293–1301. https://doi.org/10.1007/s10067-013-2443-8
Emmi G, Silvestri E, Cameli A et al (2013) Anakinra for resistant Behçet uveitis: why not? Clin Exp Rheumatol 31:152–153
Cantarini L, Vitale A, Borri M et al (2012) Successful use of canakinumab in a patient with resistant Behçet’s disease. Clin Exp Rheumatol 30:S115
Ugurlu S, Ucar D, Seyahi E et al (2012) Canakinumab in a patient with juvenile Behçet’s syndrome with refractory eye disease. Ann Rheum Dis 71:1591–1592. https://doi.org/10.1136/annrheumdis-2012-201383
Bilginer Y, Ayaz NA, Ozen S (2010) Anti-IL-1 treatment for secondary amyloidosis in an adolescent with FMF and Behçet’s disease. Clin Rheumatol 29:209–210. https://doi.org/10.1007/s10067-009-1279-8
Botsios C, Sfriso P, Furlan A et al (2008) Resistant Behçet disease responsive to anakinra. Ann Intern Med 149:284–286
Orlando I, Vitale A, Rigante D et al (2017) Long-term efficacy and safety of the interleukin-1 inhibitors anakinra and canakinumab in refractory Behçet disease uveitis and concomitant bladder papillary carcinoma. Intern Med J 47:1086–1088. https://doi.org/10.1111/imj.13538
Tugal-Tutkun I, Pavesio C, De Cordoue A et al (2018) Use of gevokizumab in patients with Behçet’s disease uveitis: an international, randomized, double-masked, placebo-controlled study and open-label extension study. Ocul Immunol Inflamm 26:1023–1033. https://doi.org/10.1080/09273948.2017.1421233
Tugal-Tutkun I, Kadayifcilar S, Khairallah M et al (2017) Safety and efficacy of gevokizumab in patients with Behçet’s disease uveitis: results of an exploratory phase 2 study. Ocul Immunol Inflamm 25:62–70. https://doi.org/10.3109/09273948.2015.1092558
Vitale A, Rigante D, Caso F et al (2017) Interleukin-1 blockade in neuro-Behçet’s disease: a case-based reflection. Int J Rheum Dis 20:1046–1049. https://doi.org/10.1111/1756-185X.12680
Gül A, Tugal-Tutkun I, Dinarello CA et al (2012) Interleukin-1β-regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of Behcet’s disease: an open-label pilot study. Ann Rheum Dis 71:563–566. https://doi.org/10.1136/annrheumdis-2011-155143
Grayson PC, Yazici Y, Merideth M et al (2017) Treatment of mucocutaneous manifestations in Behçet’s disease with anakinra: a pilot open-label study. Arthritis Res Ther. https://doi.org/10.1186/s13075-017-1222-3
Fabiani C, Vitale A, Emmi G et al (2017) Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. https://doi.org/10.1007/s10067-016-3506-4
Vitale A, Insalaco A, Sfriso P et al (2016) A snapshot on the on-label and off-label use of the interleukin-1 inhibitors in Italy among rheumatologists and pediatric rheumatologists: a nationwide multi-center retrospective observational study. Front Pharmacol. https://doi.org/10.3389/fphar.2016.00380
Emmi G, Silvestri E, Cantarini L et al (2017) Rapid desensitization to anakinra-related delayed reaction: need for a standardized protocol. J Dermatol 44:981–982. https://doi.org/10.1111/1346-8138.13619
Emmi G, Silvestri E, Squatrito D et al (2017) Long-term efficacy and safety of anakinra in a patient with Behçet’s disease and concomitant tuberculosis infection. Int J Dermatol 56:218–220. https://doi.org/10.1111/ijd.13337
Cantarini L, Talarico R, Generali E et al (2015) Safety profile of biologic agents for Behçet’s disease in a multicenter observational cohort study. Int J Rheum Dis 1–6. https://doi.org/10.1111/1756-185X.12732
Emmi G, Talarico R, Lopalco G et al (2016) Efficacy and safety profile of anti-interleukin-1 treatment in Behçet’s disease: a multicenter retrospective study. Clin Rheumatol 35:1281–1286. https://doi.org/10.1007/s10067-015-3004-0
International Study Group for Behet’s Disease (1990) Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet. https://doi.org/10.1016/0140-6736(90)92643-V
Kaneko F, Togashi A, Nomura E, Nakamura K (2014) A new diagnostic way for Behcet’s disease: skin prick with self-saliva. Genet Res Int. https://doi.org/10.1155/2014/581468
Greco A, De Virgilio A, Ralli M et al (2018) Behçet’s disease: new insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. https://doi.org/10.1016/j.autrev.2017.12.006
Yazici H, Ugurlu S, Seyahi E (2012) Behçet syndrome: is it one condition? Clin Rev Allergy Immunol 43:275–280. https://doi.org/10.1007/s12016-012-8319-x
Ma D, Zhang C, Wang R et al (2014) Etanercept in the treatment of intestinal Behcet’s disease. Cell Biochem Biophys 69:735–739. https://doi.org/10.1007/s12013-014-9860-4
Fabiani C, Sota J, Rigante D et al (2018) Efficacy of adalimumab and infliximab in recalcitrant retinal vasculitis inadequately responsive to other immunomodulatory therapies. Clin Rheumatol. https://doi.org/10.1007/s10067-018-4133-z
Deroux A, Chiquet C, Bouillet L (2015) Tocilizumab in severe and refractory Behcet’s disease: four cases and literature review. Semin Arthritis Rheum doi. https://doi.org/10.1016/j.semarthrit.2015.11.012
Acknowledgements
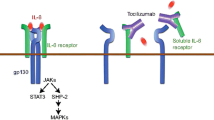
The authors wish to thank Stefano Salvati and Javier Hernández Plasencia for their help in preparing the Fig. 2.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of the work. Authors AB and GE contributed to the design of the work and in the acquisition and analysis of data. Authors ES, GDS, GL, CS, LC, AS, and GE contributed in the interpretation of data for the work. Authors AB and GE contributed in drafting the work, and all other authors revised it critically for important intellectual content. All authors approved the final version of the manuscript to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
LC received research grant and participated at speaker’s bureau by Sobi and Novartis. GE received fee from SOBI for consultancy and participated at speaker’s bureau by Novartis. AS received fee from SOBI for consultancy. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bettiol, A., Silvestri, E., Di Scala, G. et al. The right place of interleukin-1 inhibitors in the treatment of Behçet’s syndrome: a systematic review. Rheumatol Int 39, 971–990 (2019). https://doi.org/10.1007/s00296-019-04259-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04259-y