Abstract
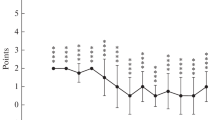
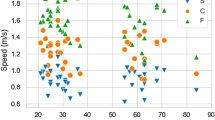
It is well established that arthritis depresses locomotion in humans as well as in animal disease models. The K/BxN mouse model resembles rheumatoid arthritis and is widely used for research. Here, we investigate the behavioral alterations of arthritic K/BxN mice during arthritis development with respect to horizontal locomotion. Locomotor activity measurements and the methodology of ankle thickness measurements are compared to demonstrate the feasibility of motion tracking in the K/BxN mouse model. Arthritic K/BxN mice show significantly decreased locomotion compared to their non-arthritis K/BxN littermates. We found an indirect correlation of ankle thickness and locomotor activity. However, both parameters are only partially interdependent resulting in temporal displacement of maximal ankle swelling and maximal depression of locomotion by 1 week. Assessing the impaired movement as a behavioral test appears to be a valuable multifactorial parameter for the evaluation of arthritis in the K/BxN mouse model and provides additional information on disease progression and severity.





Similar content being viewed by others
References
Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D (1996) Organ-specific disease provoked by systemic autoimmunity. Cell 87:811–822
Matsumoto I (1999) Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 286:1732–1735
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Jonsson B, Larsson SE (1990) Rheumatoid arthritis evaluated by locomotion score: a population study. Scand J Rheumatol 19:223–231
Jonsson B, Larsson SE (1990) Hand function and total locomotion status in rheumatoid arthritis: an epidemiologic study. Acta Orthop Scand 61:339–343
Larsson SE, Jonsson B (1989) Locomotion score in rheumatoid arthritis. Acta Orthop Scand 60:271–277
Christianson CA, Corr M, Firestein GS, Mobargha A, Yaksh T, Svensson CI (2010) Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain 151:394–403
Mrosovsky N (1996) Locomotor activity and non-photic influences on circadian clocks. Biol Rev 71:343–372
Sasakawa T, Sasakawa Y, Ohkubo Y, Mutoh S (2005) FK506 ameliorates spontaneous locomotor activity in collagen-induced arthritis: implication of distinct effect from suppression of inflammation. Int Immunopharmacol 5:503–510
Hartog A, Hulsman J, Garssen J (2009) Locomotion and muscle mass measures in a murine model of collagen-induced arthritis. BMC Musculoskel Dis 10:59
Solomon S, Kolb C, Mohanty S, Jeisy-Walder E, Preyer R, Schöllhorn V, Illges H (2002) Transmission of antibody-induced arthritis is independent of complement component 4 (C4) and the complement receptors 1 and 2 (CD21/35). Eur J Immunol 10:644–651
Correll N, Sempo G, de Meneses YL, Halloy J, Deneubourg JL, Martinoli A (2006) SwisTrack: a tracking tool for multi-unit robotic and biological systems. IEEE/RSJ Int Conf Intell Robots Syst 2185–2191
Boettger MK, Hensellek S, Richter F, Gajda M, Stöckigt R, von Banchet GS, Bräuer R, Schaible H (2008) Antinociceptive effects of tumor necrosis factor α neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum 58:2368–2378
Millecamps M, Jourdan D, Leger S, Etienne M, Eschalier A, Ardid D (2005) Circadian pattern of spontaneous behavior in monoarthritic rats: a novel global approach to evaluation of chronic pain and treatment effectiveness. Arthritis Rheum 52:3470–3478
Carlsson M, Carlsson A (1990) Interactions between glutamatergic and monoaminergic systems within the basal ganglia-implications for schizophrenia and Parkinson’s disease. Trends Neurosci 13:272–274 (C1–C4, 275–276)
Good RL, Radcliffe RA (2011) Methamphetamine-induced locomotor changes are dependent on age, dose and genotype. Pharmacol Biochem Behav 98:101–111
Nikolaidis N, Kissinger C (2006) Nicotine-induced chances in core body temperature and locomotor activity in conscious and freely-moving rats. Curr Separat Drug Dev 21:79–81
Acknowledgments
We would like to thank the Distributed Intelligent System and Algorithms Laboratory (DISAL) and the École Polythechnique Fédéral de Lausanne for sharing the SwisTrack software. Furthermore, we want to thank Christian Schink for technical support as well as Martian Krämer and Debra Greyson for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frommholz, D., Illges, H. Maximal locomotor depression follows maximal ankle swelling during the progression of arthritis in K/BxN mice. Rheumatol Int 32, 3999–4003 (2012). https://doi.org/10.1007/s00296-011-2337-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-011-2337-5