Abstract
For long time, studies on ectomycorrhiza (ECM) have been limited by inefficient expression of fluorescent proteins (FPs) in the fungal partner. To convert this situation, we have evaluated the basic requirements of FP expression in the model ECM homobasidiomycete Laccaria bicolor and established eGFP and mCherry as functional FP markers. Comparison of intron-containing and intronless FP-expression cassettes confirmed that intron-processing is indispensable for efficient FP expression in Laccaria. Nuclear FP localization was obtained via in-frame fusion of FPs between the intron-containing genomic gene sequences of Laccaria histone H2B, while cytosolic FP expression was produced by incorporating the intron-containing 5′ fragment of the glyceraldehyde-3-phosphate dehydrogenase encoding gene. In addition, we have characterized the consensus Kozak sequence of strongly expressed genes in Laccaria and demonstrated its boosting effect on transgene mRNA accumulation. Based on these results, an Agrobacterium-mediated transformation compatible plasmid set was designed for easy use of FPs in Laccaria. The four cloning plasmids presented here allow fast and highly flexible construction of C-terminal in-frame fusions between the sequences of interest and the two FPs, expressed either from the endogenous gene promoter, allowing thus evaluation of the native regulation modes of the gene under study, or alternatively, from the constitutive Agaricus bisporus gpdII promoter for enhanced cellular protein localization assays. The molecular tools described here for cell-biological studies in Laccaria can also be exploited in studies of other biotrophic or saprotrophic basidiomycete species susceptible to genetic transformation.
Similar content being viewed by others
Introduction
Fluorescent proteins (FP) are molecular markers, which through their light emission allow monitoring cellular processes and responses in vivo. Expression of FPs fused with protein-coding or gene regulatory sequences allows live-cell imaging to study, e.g., cellular protein location, protein dynamics and interactions, gene expression activities, and to visualize whole organelles and different functional cell compartments (Chudakov et al. 2010). FPs are widely utilized cellular markers in fungal research and numerous species from different phyla have been genetically modified to express green fluorescent protein (GFP) (Prasher et al. 1992), its different enhanced sequence mutants (eGFPs) and other FPs. During the last 20 years, the use of fluorescent markers has provided vast amounts of information covering variable aspects of fungal biology, such as visualization of fungal cells when interacting with plant tissue (Spellig et al. 1996; Vanden Wymelenberg et al. 1997; Kilaru et al. 2015) as well as revealing the importance of specific fungal proteins and cellular processes during plant–pathogen interactions (Doehlemann et al. 2009; Khang et al. 2010; Dagdas et al. 2012; Bielska et al. 2014; Lanver et al. 2017; Shipman et al. 2017). However, the specific codon usage is a limiting factor for efficient FP expression in some fungi. Therefore, codon-optimized synthetic FPs were designed to successfully express FPs in some fungal species such Candida albicans, Botrytis cinerea, and Zymoseptoria tritici (Cormack et al. 1997; Leroch et al. 2011; Kilaru et al. 2015). In addition, especially in some basidiomycete species, efficient FP expression is highly intron processing-dependent (e.g., Burns et al. 2005; Ford et al. 2016).
Laccaria bicolor is a homobasidiomycete, which forms ectomycorrhiza (ECM) with several boreal and temperate forest trees such as birch, pine, and poplar. ECM is a symbiotic interaction between soil fungi and tree roots and its function is fundamental, not only for survival of the symbiotic fungi in soil, but for tree growth and nutrition in forest ecosystems (Smith and Reed 2008; Martin et al. 2016). Among ECM basidiomycetes, L. bicolor is an excellent model for genetic studies of ECM; the genome of the fungus is fully sequenced (Martin et al. 2008), Laccaria is susceptible to genetic modification via Agrobacterium tumefaciens-mediated transformation (ATMT) (Kemppainen et al. 2005), and its gene expression can be altered via RNA silencing (Kemppainen et al. 2009; Kemppainen and Pardo 2010). During the last 10 years, a vast amount of genetic information has been generated on the control and functionality of Laccaria–plant host interaction. These studies have revealed, among many, that similar to establishment of pathogenic interaction, formation of ECM is mediated by secreted fungal and plant effector molecules, and the symbiotic interaction results in modification of plant defense responses and hormone metabolism (Martin et al. 2008; Plett et al. 2011, 2014, 2015, 2017; Vayssières et al. 2015).
The importance of FPs for deeper understanding of plant–fungus pathogenesis is undeniable and similar benefits from FP marker expression for symbiotic interaction studies are to be expected. Unfortunately, previous attempts to achieve efficient FP expression in Laccaria had failed (Kemppainen et al. 2005, Kemppainen unpublished results), leaving thus an important gap in the repertoire of molecular tools available for Laccaria and ECM research. Two potential reasons explaining this failure include unfit codon usage and/or lack of intron processing, as the sGFP(S65T) used in those experiments has human codon optimization and it lacks introns. However, this GFP variant has been successfully expressed in another basidiomycete, namely the dimorphic yeast Ustilago maydis (Spellig et al. 1996).
Therefore, the main goals of the work presented here were: (1) to study the basic requirements of FP expression in Laccaria and (2) to establish both GFP and the red fluorescent protein mCherry as functional FP markers for the fungus. A nucleus-directed approach was applied to evaluate the need of intron processing for successful FP expression in Laccaria. Nuclear FP targeting is expected to produce highly localized and concentrated fluorescent signals in the cell, facilitating thus FP signal detection. The Laccaria nucleosome core histone H2B family was selected for testing this intron-dependency of FP expression as its sequences harbor introns (Yun and Nishida 2011). Histone-FP fusions are demonstrated to become incorporated into the nucleosomes similarly to native histone proteins (Kanda et al. 1998), and therefore, these are expected to localize in nucleus also through all mitotic phases in the cells. Histone H2B, like other nucleosome core histones (H1, H2A, H3, and H4) and the linker histone H1, are represented in plants, animals, and humans by multiple isoforms (Molden et al. 2015; Jiang and Berger 2017). There are both replication-dependent, canonical, histones that are expressed throughout the S phase of the cell cycle and which are often clustered in the genome, and replication-independent histones that show tissue-specific or stimuli-induced expression. Successful expressions of canonical histone H2B–FP fusions are reported in animals (Kanda et al. 1998; Hadjantonakis and Papaioannou 2004; Fraser et al. 2005), plants (Boisnard-Lorig et al. 2001; Rosa et al. 2014), and in fungi (Maruyama et al. 2001; Rech et al. 2007; Walter et al. 2010; Ding et al. 2016).
Also the presence of the Kozak sequence at the 5′ UTR of mRNAs can influence the efficiency of transgene expression in eukaryotes, not only by affecting translation but also optimal transcription and mRNA stability (Kozak 1987; Le Hir et al. 2003; Haruyama et al. 2009; Jackson et al. 2010; Gallegos and Rose 2015). In eukaryotes, translation initiation involves start codon recognition by ribosomes via a mechanism called ribosome scanning (for a review see Hinnebusch 2017). This is strongly dependent on the Kozak sequence, a sequence motif, which overlaps with the start codon itself. The first eukaryotic Kozak consensus sequence, (gcc)gccRccATGG, was established based on vertebrate genes (Kozak 1984), and especially, the − 3 purine and the + 4 guanine of this sequence (A in ATG is considered the position + 1) were shown highly important for the translation strength. As the Kozak sequence varies to some extent among eukaryotes, the consensus Kozak sequence, established based on vertebrate genes, can be a weak predictor of translation efficiency in other eukaryotes (Grzegorski et al. 2014). Therefore, an optimal Kozak sequence should be evaluated in each organism under study. Generally, no special attention has been paid on incorporation of Kozak sequences into the transgene expression cassettes in basidiomycete studies, and both the specific Kozak consensus sequence features and its effect on translation efficiency in filamentous basidiomycetes are currently very poorly investigated. Therefore, we have evaluated the effects of an optimized Kozak sequence on transgene expression in Laccaria.
As a result of these FP expression studies, we present a plasmid toolkit for flexile and ATMT-compatible use of FPs in Laccaria. Even though different plasmid systems exist for seamless use of FPs in filamentous ascomycetes (Toews et al. 2004; Schoberle et al. 2013; Gong et al. 2015; Nishikawa et al. 2016), similar molecular tools adapted for basidiomycetes are scarce, limiting thus the use of FP markers in these fungi. To our knowledge, the plasmid set described here is the first ATMT–compatible FP fusion toolkit, which is specifically designed for homobasidiomycete studies. Even though designed to be used with ATMT methodology, these vectors are naturally adaptable to any other fungal transformation methodology reliant on use of plasmid DNA, and are, therefore, a novel and flexible tool, not only for ECM studies, but also for filamentous basidiomycetes in general.
Materials and methods
Strains, media, and growth conditions
Laccaria bicolor (Maire) Orton dikaryotic wild-type strain S238N (Di Battista et al. 1996) and the monokaryotic strain S238N-H82 were maintained on solid-modified P5 medium (MP5) (Kemppainen et al. 2008) at 22 °C in darkness. For genomic DNA extraction, fungal mycelium was cultivated under the same growth conditions on sterile cellophane membrane covered solid medium plates for 10 days (Couvre-Confitures, Hutchinson, Chalette sur Loing, France). Genetically modified Laccaria strains were maintained on MP5 supplemented with 150 µg/ml of hygromycin B (Invitrogen, Thermo Fisher Scientific, USA). Escherichia coli strain TOP10 (Invitrogen) was used for all molecular cloning and plasmid replication steps and hypervirulent Agrobacterium tumefaciens strain AGL1 (Lazo et al. 1991) in A. tumefaciens-mediated transformation of Laccaria. The plasmid constructs were introduced into E. coli and A. tumefaciens by a standard electroporation protocol (Sambrook et al. 1989). During the plasmid cloning steps, E. coli was cultivated on Luria–Bertani (LB) medium supplemented with the required antibiotics (100 µg/ml ampicillin, 100 µg/ml kanamycin, or both) at 37 °C. The strain AGL1 carrying the binary vectors was grown on LB medium, supplemented with 100 µg/ml kanamycin, at 28 °C.
Plasmid constructs
The plasmid cloning steps were completed using standard restriction enzyme—T4 DNA ligase-based methods (Sambrook et al. 1989). The molecular-cloning enzymes and reagents were purchased from Promega (USA), Takara Bio USA, Inc (USA) or Productos Bio-Lógicos (Argentina), while the gDNA extraction kit (DNAeasy plant mini kit), and the agarose gel DNA extraction kit (QIAquick Gel Extraction Kit) were provided by Quiagen (Germany). The PCR reactions were performed with a proofreader DNA polymerase (AccuPrime Pfx DNA Polymerase, Invitrogen) according to the manufacturer’s instructions using a TPersonal thermocycler (Biometra, Germany). All oligonucleotides used for plasmid construction (Online Resource file 1; Table S1) were purchased from Genbiotech SRL (Argentina) and sequencing services were obtained from Macrogen (South-Korea).
The vectors pHTB16202GFP and pHTB16202mCherry were constructed using pSILBAγ (Kemppainen and Pardo 2010) as initial cloning platform. These two plasmids carry the Laccaria histone H2B genomic gene sequence (JGIv2 ID #185040, gene HTB16202, Laccaria bicolor genome assembly v2.0 https://genome.jgi.doe.gov/Lacbi2/Lacbi2.home.html), together with its native regulatory region (1000 bp upstream of ATG), translationally fused at its C-terminus either to eGFP (Chiu et al. 1996) or to mCherry (Shaner et al. 2004) fluorescent protein encoding genes. Initially, the A. bisporus gpdII promoter, together with the multiple cloning sites I and II, was removed from pSILBAγ with BamHI cut and replaced by eGFP or mCherry gene sequences, which were amplified with primers BamHISmaILINKgfp-F/BglIISTOPgfp-R and BamHISmaILINKgfp-F/BglIISTOPmCherry-R from pBGgHg (Chen et al. 2000) and pmCherry-N1 (Clontech Laboratories, Inc. USA) plasmid templates. This generated the intermediate plasmids pLink-GFP and pLink-mCherry, respectively. The genomic gene sequence of Laccaria histone H2B (isoform HTB16202), excluding the stop codon and including 1000 bp upstream of the start codon, was amplified with primers BamHIXbaIH2BProm-F/SmaIH2B-R from gDNA of the dikaryotic Laccaria strain S238N. The BamHI-SmaI digested gene amplicon was ligated at its 3ʹ in-frame with eGFP or mCherry in pLink-GFP and pLink-mCherry to create the final plasmid constructs pHTB16202GFP and pHTB16202mCherry. These in-frame gene fusions contained a five amino acid linker sequence Pro–Gly–Ile–Ala–Gly (CCC GGG ATT GCA GGT) (Fig. 2c). The H2B-FP expression cassettes in pHTB16202GFP and pHTB16202mCherry were terminated by Aspergillus nidulans tryptophan biosynthesis gene terminator, TtrpC, originally present in pSILBAγ.
The cloning plasmids pN-GFP and pN-mCherry were constructed by modifying pLink-GFP and pLink-mCherry vectors. Initially, the Laccaria nitrate reductase intron was removed from the multiple cloning site of pSILBAγ as HindIII-BglII fragment, and replaced by a dsDNA oligo linker (annealed with oligo1HindIII-EcoRI and oligo2BglII-EcoRI, Online Resource file 1; Table S1), which introduced a novel EcoRI site. This modified MCS in pSILBAγ was excised, together with the A. bisporus gpdII promoter of pSILBAγ as BamHI fragment and cloned in reverse orientation to the BamHI cut pLink-GFP and pLink-mCherry to form the final FP fusion cloning plasmids pN-GFP and pN-mCherry, respectively.
Similarly, plasmids pCEBN-GFP and pCEBN-mCherry were constructed using pLink-GFP and pLink-mCherry intermediate vectors via introducing the modified EcoRI containing MCS of pSILBAγ, together with the A. bisporus gpdII promoter as a BamHI fragment. The relevant sequences of pN-GFP, pN-mCherry, pCEBN-GFP, and pCEBN-mCherry for FP fusion cloning use [sequences between the M13/pUC sequencing primer (-20), 17-mer and the M13/pUC sequencing primer (-26), 17-mer] are available in Online Resource file 2 and deposited in GenBank (Accessions numbers pN-mCherry: MN781138, pN-GFP: MN781139, pCEBN-mCherry: MN781140, and pCEBN-GFP: MN781141).
To test the functionality of pCEBN-GFP and pCEBN-mCherry, the genomic gene sequence of Laccaria H2B (HTB16202), lacking its stop codon and including either the native Kozak sequence (9 nt upstream of ATG) or the Kozak of Laccaria glyceraldehyde 3-phosphate dehydrogenase gene (GPD) (JGIv2 ID #318873), was cloned as a XhoI-SmaI fragment translationally in-frame with the fluorescent protein-encoding genes. The amplification of the Laccaria histone H2B isoform HTB16202 was carried out with primers H2BXhoINativeKozak-F or H2BXhoIgpdKozak-F, in combination with SmaIH2B-R, using pHTB16202GFP as PCR template. The products of this cloning step were the plasmids pCEBN-GFP/KNH2B, pCEBN-GFP/KgpdH2B, pCEBN-mCherry/KNH2B, and pCEBN-mCherry/KgpdH2B.
For launching constitutive cytosolic expression of GFP and mCherry in Laccaria, the first 204 nucleotides of Laccaria glyceraldehyde 3-phosphate dehydrogenase gene (GPD) (JGIv2 ID #318873, the manually curated GPD gene model), including its native Kozak sequence (9 nt upstream of ATG), were cloned in-frame with the fluorescent protein-encoding genes in pCEBN-GFP and pCEBN-mCherry. The 5′ fragment of the GPD was amplified from gDNA of the monokaryotic strain S238N-H82 with primers GPDintronsXhoI-F and GPDintronsSmaI-R. The resultant plasmids were named pCEBN-cytosolGFP and pCEBN-cytosolmCherry. The control vectors, pCEBN-GFPmod and pCEBN-mCherrymod, used for assessing the intron dependence of FP expression in Laccaria, were prepared by digesting pCEBN-GFP and pCEBN-mCherry with SnaBI and SmaI and self-ligated to eliminate part of their MCS.
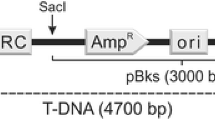
For the ATMT of Laccaria, all the plasmid vectors described above were SacI linearized and cloned as full plasmids into the SacI site in the T-DNA of Agrobacterium binary vector pHg (Kemppainen and Pardo 2010).
Fungal transformation
Transformation of Laccaria dikaryotic strain S238N with Agrobacterium tumefaciens strain AGL-1 was carried out as previously described (Kemppainen et al. 2005), with the following modifications: The minimum medium cultivation of AGL1 was excluded and 1 ml Agrobacterium LB cultures grown overnight in 1.5 ml Eppendorf tubes were directly used for acetosyringone (AS) induction. Before this 6 h induction, bacterial cells were centrifuged at 5000 rpm at 4 °C for 10 min, the LB supernatant was removed, and the cell pellet was re-suspended in identical volume of the induction medium containing AS (final concentration of AS 200 µM). Laccaria was co-cultivated with Agrobacterium at 22 °C for 4 days, after which fungal colonies were plated for selection on MP5 medium supplemented with 600 µg/ml ceftriaxone and 300 µg/ml hygromycin B. After 2 weeks of primary selection, a set of 48 randomly selected hygromycin B-resistant fungal growth points were passed through two consecutive, 1 week each, selection rounds on MP5 supplemented with 200 µg/ml ceftriaxone and 300 µg/ml hygromycin B. The final transformation efficiency (calculated as hygromycin B resistant growth points per fungal colonies subjected to co-cultivation) was assessed for each transformation construct after 3 weeks of primary selection.
Fluorescence microscopy
For fluorescence microscopy analysis, small fragments of fungal mycelia, cut from colony borders grown on solid MP5 medium, were mounted on microscope slides in liquid MP5 medium. For DAPI staining, mycelia was mounted in MP5 medium with 0.5 µg/ml DAPI (Sigma-Aldrich, USA) and observed after 10 min of incubation at RT. Bright field and fluorescence microcopy were carried out with a Leica DMI6000B inverted microscope using an external mercury metal halide bulb light source (Leica Küblercodix EL6000) for fluorescence excitation. The filter cubes for DAPI, eGFP, and mCherry signal detection were cubes A, GFP, and N2.1, respectively. The images were captured with a high-resolution digital monochrome camera (Leica DFC345 FX) and processed with Leica Application Suite V3.8.0 software (Leica Microsystems, Germany). The photo editing (image coloring, contrast adjustment, and merging) was done with Fiji ImageJ software (Schindelin et al. 2012).
Analysis of Laccaria consensus Kozak sequence
Public transcriptomic data of L. bicolor on Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/) were used for constructing the consensus Kozak sequence present in highly expressed genes of the fungus. The GEO accession GSE35556 (Gene expression of Laccaria bicolor S238N free-living mycelium grown on different media—GPL14641 INRA 4-plex Laccaria bicolor whole-genome expression array) data sets GSM870439, GSM870440, and GSM870441(free-living mycelium of Laccaria bicolor S238N grown on MMN medium for 10 days, three biological replicates) were used for detecting the most highly expressed genes in Laccaria (Online Resource file 4). The 50 most highly expressed gene models of dataset GSM870439 (44 of which were also present among the 50 most expressed genes in one or both of the other two biological replicas), and the models shared between replicas GSM870440 and GSM870441, but not detected among the most highly expressed ones in GSM870439 (4 models), were used for calculating the mean expression values of 54 gene models, altogether. The Laccaria protein IDs (JGI genome version 1.0) corresponding to these NimbleGen gene model IDs were extracted from the design data of the GPL14641 genomic chip array and the sequences of the genes were localized at Laccaria genome v1.0 portal (https://genome.jgi.doe.gov/Lacbi1/Lacbi1.home.html). The predicted transcript sequences of the JGIv1.0 gene models were used to further identify corresponding updated Laccaria genome v2.0 gene models (https://genome.jgi.doe.gov/Lacbi2/Lacbi2.home.html) using Blastn searches against the filtered model transcript database. The JGIv2.0 gene models were evaluated for their transcriptomic status (full-coverage Sanger or 454 EST support and/or Illumina EST-derived gene model). As a result, 47 genes, among which 15 manually annotated genes, were used in consensus Kozak sequence analysis of highly expressed genes of Laccaria. Fifteen nucleotides (10 nt upstream and 2 nt downstream of ATG) were extracted from the genomic gene sequences and used for final-sequence logo detection at WebLogo portal (https://weblogo.berkeley.edu/). The consensus Kozak sequence was manually constructed using nucleotide frequencies (%) at each of the 15 sequence sites analyzed. The cut-off for a conserved nucleotide was set to ≥ 40% and the absence of a specific nucleotide to ≤ 10% at each site analyzed (Online Resource file 3).
Assessing the effect of Laccaria consensus Kozak sequence on transgene expression
Laccaria pectin methyl esterase-encoding gene LbPME1 (JGIv2 ID #245379) was used as a test gene for evaluating the effects of the Kozak sequence on transgene overexpression. The native Kozak sequence of LbPME1 shows characteristics of a weakly translated Kozak sequence with cytosine three nucleotides upstream of ATG (e.g., position − 3 nt). Therefore, two LbPME1 overexpression constructs were prepared using pSILBAγ cloning vector (Kemppainen and Pardo 2010). One contained the genomic gene sequence, including the native gene Kozak sequence (9 nt upstream of ATG: 5ʹ -TCTTTTCAAATG) and the other 9 nt upstream of Laccaria glyceraldehyde 3-phosphate dehydrogenase gene (JGIv2 ID #318873; 5ʹ-ACTATCACAATG), which fulfills the characteristics of the consensus Kozak sequence of highly transcribed genes in Laccaria. The LbPME1 was amplified from gDNA of Laccaria using primers PME1NatKozSnaBI-F and PME1StopSphI-R or o PME1gpdKozSnaBI-F and PME1StopSphI-R (Online Resource file 1; Table S1). The gene sequences were cloned into pSILBAγ, under the constitutive A. bisporud gpdII promoter, using SnaBI and SphI sites. The LbPME1 expression cassettes were further introduced as full SacI linearized vectors into the SacI site in pHg (Kemppainen and Pardo 2010) and used for ATMT. Twelve randomly selected transformants produced with each of the two overexpression vectors were analyzed for the LbPME1 transcript levels by qRT-PCR.
RNA extraction, cDNA synthesis, and qRT-PCR
Free-living mycelia of transgenic lines and wild-type fungus were grown on cellophane membranes placed on modified low-glucose Pachlewski agar (P20) medium (as MP5 but 1 g glucose/l), in the case of transformant lines supplemented with 300 µg/ml hygromycin B, at 20 °C in the dark. Mycelial samples harvested at 14 days under RNase-free conditions were flash-frozen in liquid nitrogen and stored at − 80 °C freezer before RNA extraction. Samples of approximately 100 mg were ground with a Retsch Mixer Mill under cryogenic conditions. RNA was extracted from the finely ground samples with RNeasy plant Mini Kit (Qiagen, Cat No. 74904) following the manufacturer’s protocols together with recommended on-column digestion of DNA with RNase-Free DNase Set (Qiagen, Cat No. 79254). An additional DNA digestion step was performed in the total RNA samples using DNA-free™ DNA Removal Kit (Invitrogen, Cat No. AM1906) according to the manufacturer’s instructions followed by RNA cleanup using RNeasy MinElute Cleanup Kit (Qiagen, Cat No. 74204). Total RNA concentrations were measured with a NanoDrop and adjusted to 100 ng/µl. RNA integrity was confirmed by agarose gel electrophoresis before cDNA synthesis. First-strand cDNA was synthesized from 200 ng total RNA using the iScript cDNA synthesis kit (Bio-Rad, USA) following the manufacturer’s instructions. Quality of cDNAs was checked with regular PCR using ‘Ubiquitin’ primers (Online Resource file 1; Table S2).
The qRT-PCR was performed to determine the relative expression level of the targeted gene of interest LbPME1 in the overexpression lines. The gene-specific qRT-PCR primers were designed using ‘QuantPrime’ web application (Arvidsson et al. 2008) from the L. bicolor transcriptome. The efficiency of all primers used (described in Online Resource file; Table S2) was determined to be between 95 and 105% on a five-point cDNA dilution series by qRT-PCR. qRT-PCR reactions were performed in the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA) using the LightCycler® 480 SYBR Green (Roche Life Science, Cat. No. 04707516001) in a 15 μl reaction containing 7.5 μl of Sybr Green master mix, 2 μl cDNA template (diluted in a 1:5 ratio), and 10 pmol of each primer. The PCR conditions were as follows: 95 °C for 3 min, 40 cycles of 95 °C for 10 s, 55 °C for 10 s, and 72 °C for 10 s. The reaction was completed with a melt curve: 65 °C to 95 °C heating in 0.5 °C increments. Two technical replicates were performed for all samples. Relative expression of target genes was calculated against wild-type control and normalized against four stably expressed housekeeping genes: histone H4 (JGIv2 ID #319764), ubiquitin (JGIv2 ID #446085), elongation factor 3 (JGIv2 ID #659644), and metalloprotease (JGIv2 ID #245379) with ΔΔCq method (Livak and Schmittgen 2001) using CFX Maestro™ Software (Bio-Rad, USA). The statistical significance of LbPME1 overexpression in transgenic lines was calculated against wild-type expression values with Student´s t test (p < 0.05).
Results
Laccaria histone H2B as model for FP expression
As Laccaria codon usage does not significantly differ from U. maydis (evaluated based on the codon usage database https://www.kazusa.or.jp/codon/, Online Resource file 3), this let us to hypothesize, that most likely the limiting factor in Laccaria FP expression was linked to the lack of intron processing of the transgene in this basidiomycete. We selected the Laccaria nucleosome core histone H2B family for testing the intron dependence of FP expression. To assure strong, and at its best, constitutive nuclear FP localization, the full Laccaria H2B gene repertoire was screened for highly expressed replication-dependent H2B variants. We detected in the Laccaria genome five gene models sharing sequence homology with ascomycete and basidiomycete H2Bs (Online Resource file 3). To evaluate if this high number of putative H2B genes in Laccaria is a species-specific feature, a characteristic of basidiomycetes in general, or if it could even be linked to symbiotic fungal lifestyle, H2B searches were conducted on 20 randomly selected basidiomycete genomes. These included species of Agaricomycotina, Pucciniomicotina, and Ustilaginomycotina presenting different lifestyle strategies (saprotroph, ECM symbiont or pathogen). The results confirmed the elevated, but also variable number (2–6) of putative H2B genes in both Agaricomycotina and Pucciniomicotina, while Ustilaginomycotina appears to have only one H2B isoform. However, the number of H2B genes, especially among Agaricomycotina species, did not show any evident fungal lifestyle linkage, suggesting that the elevated number of H2B isoforms is a taxon-specific feature of basidiomycetes in general (Online Resource file 3).
Among these five putative Laccaria histone H2B gene models, two are manually curated as H2Bs (genes HTB16201 and HTB16202) and these count with full transcript coverage confirmation (Laccaria genome JGIv2.0). Based on the analysis conducted using the open access Laccaria full-genome array hybridization data (Geo Accession Series GSE35556, Online Resource file 3), both HTB16201 and HTB16202 are strongly and at similar level expressed in free-living mycelium and their expression strength is equivalent to constitutive structural genes, such as beta-actin. On the contrary, the three other Laccaria H2B gene models are supported by only very weak and partial EST data and, concordantly, their expression levels were also found minimal (Online Resource file 3). These data suggest HTB16201 and HTB16202 as putative replication-dependent canonical histone H2Bs, responsible for nucleosome assembly in mitotic cells, while the three other H2B gene models could either represent replication-independent H2B variants of the fungus or be pseudogenes. In addition, both HTB16201 and HTB16202 are located in close vicinity of H2A genes (H2A isoform-encoding genes HTA16203 and HTA16205, respectively) (Fig. 1). At these histone loci, the H2A and H2B genes are placed transcriptionally in opposite directions and separated by 196 nt, a feature that indicates a co-regulated expression mode of the H2A and H2B histones in Laccaria. Even though no cell cycle-linked expression profiles, which would confirm their expression specifically in the S phase of cell division, are available for HTB16201 and HTB16202, the high general transcriptional levels in vegetative mycelium, together with the conserved genome organization, were taken as confirmation of the functional role of HTB16201 and HTB16202 as canonical replication-dependent histone isoforms of Laccaria. Consequently, one of these genes, HTB16202, was arbitrarily selected as a test gene for nuclear FP localization in Laccaria.
Laccaria has two H2B-H2A histone loci, in which the canonical, replication-dependent H2B and H2A isoform gene pairs, HTB16201-HTA16203 and HTB16202-HTA16205, respectively, are located very close to each other. These gene pairs are organized in a transcriptionally inverted manner and thus apparently share important regulatory sequences. The exact genomic locations of the genes are indicated in parenthesis
The Laccaria histone H2B isoform HTB16202 consists of 4 exonic and 3 intronic sequences, and it also has confirmed 5´UTR and 3´UTR sequences (Fig. 1, the gene sequence data are available in Online Resource file 3). HTB16202 encodes a predicted H2B protein of 145 amino acids, and structurally, this is expected to consist of a globular central H2B fold domain and nucleosome protruding flexible 54 aa N-terminal tail (Fig. 2a), the two basic structures present in all the four core nucleosome histone proteins in eukaryotes (Luger 2006). However, among the core histones, H2A and H2B also contain a shorter C-terminal tail, which in HTB16202 is of 16 aa (Fig. 2a) (the amino acid sequence homology-based analysis of the protein structure is available in the Online Resource file 3). As post-translational modification (PTM) has a fundamental role in variable regulatory effects on gene transcription, chromosome stability, and DNA repair, we examined the presence of sites for PTM in HTB16202. To do so, we compared Laccaria HTB16202 with its closely homologous Saccharomyces cerevisiae H2B isoforms, HTB1, and HTB2 (72.1% and 74% sequence identity, respectively), and used the existing information on PTMs affecting Saccharomyces H2Bs (Online Resource file 3). Conserved PTM target amino acids for potential acetylation, phosphorylation, succinylation, methylation, dimethylation, glycosylation, and ubiquitination were detected both in N-and C-terminal tails, as well as in the central histone fold domain, the N-terminal tail being, however, potentially most PTM targeted part of this Laccaria histone. As the majority of the predicted PTMs were located at the N-terminal tail of HTB16202, we decided for a C-terminal FP-marker fusion approach for ATMT (Fig. 2b), to avoid interfering with histone-mediated chromatin modification processes and, consequently, to reduce the risk of affecting viability or the environmental responsiveness of the Laccaria H2B-FP transformants.
aLaccaria histone H2B (HTB16202) is a predicted mature protein of 145 amino acids with typical histone H2B structural features; N-terminal tail, central histone H2B fold, and C-terminal tail. b Histone H2B forms part of the nucleosome core, being present as duplicate in each nucleosome and forming a protein pair with histone H2A. The long N-terminal tail as well as the short C-terminal tail of H2B protrude outwards from the nucleosome core and especially the N-terminal tail is known to go through variable post-translationally modifications involved in variable aspect of gene expression regulation in eukaryotes (i.e., histone code). Histone–FP fusion proteins are demonstrated to get incorporated as structural parts of nucleosomes. c Schematic presentation of the H2B-FP fusion expression cassettes pHTB16202GFP and pHTB16202mCherry used for nuclear FP localization in Laccaria. The fusion protein expression was driven from the endogenous HTB16202 promoter sequence (1000 bp) and the expression cassettes were terminated by the Aspergillus nidulans trpC terminator. ex1–4 refer to exons in HTB16202
HTB16202–FP fusion constructs and nuclear FP expression in Laccaria
Two FP marker genes were tested in Laccaria: (1) Aequorea victoria green fluorescent protein enhanced synthetic variant sGFP(S65T) (ex/em 489/509) (Chiu et al. 1996), and (2) the red fluorescent protein mCherry (ex/em 587/610) (Shaner et al. 2004), the latter being reported highly stable and resistant to photobleaching. The HTB16202 genomic gene sequence, without the stop codon, and including its upstream regulatory sequences were cloned in-frame with the two FP genes (Fig. 2c). The endogenous promoter of a canonical H2B should produce strong, but strictly cell cycle regulated expression of the H2B–FP fusion proteins. However, the reported high stability of histone proteins was expected to compensate for this temporal transcriptional variation and produce stable nuclear FP localization in Laccaria. Even though the ORF of HTB16202 is separated from its neighboring H2A isoform-encoding gene (HTA16205) only by 354 bp (Fig. 1), suggesting that the necessary regulatory elements for this histone H2B isoform expression could already be located within this short sequence; 1000 bp upstream of the HTB16202 start codon were used for running the fusion protein expression. The C-terminal translational fusion was constructed using a 5 amino acid non-polar linker (i.e., PGIAP) to assure proper folding of the fused protein partners and the H2B-FP expression cassette vectors pHTB16202GFP and pHTB16202mCherry (Fig. 2c) were used for ATMT of Laccaria dikaryotic strain, once incorporated into the T-DNA of the vector pHg (Kemppainen and Pardo 2010).
The ATMT with both H2B-FP vectors resulted in very high transformation efficiency (260% for pHTB16202GFP and 280% for pHTB16202mCherry, respectively) and in production of primary libraries of 197 and 199 hygromycin B-resistant fungal lines. Twelve randomly selected transformants from each H2B-FP expression library were evaluated for FP signals by fluorescence microscopy. The vast majority of the fungal lines observed (H2B-GFP: 9/12 and H2B-mCherry: 11/12) showed intense, localized fluorescent signals within their cells. DAPI staining co-localized with this signal confirmed successful nuclear FP localization with both eGFP and mCherry in Laccaria (Fig. 3). Particularly, intense FP signals were observed in mitotic cells with condensed nuclear material. The expression of H2B-FP fusion did not significantly affect fungal growth (Fig. 4a–c), and most importantly, the fluorescent signal was stable in time without hygromycin B selection. Transformants grown without the antibiotic in the culture medium for 1 month continued to show strong nuclear FP localization (Fig. 4d, e). Even though in such aged mycelia, the cell wall autofluorescence is increased, the intensity of the nuclear H2B-FP signal overcame it greatly. Between the two FPs tested, mCherry, performed undoubtedly better than sGFP(S65T), resulting in longer lasting fluorescent signals due to higher resistance to photobleaching. Moreover, the hyphal autofluorescence, especially in young mycelia was clearly lower within the detection wavelength range of mCherry compared to GFP, a factor, which strongly promotes mCherry as the FP marker of choice for Laccaria. The fact that robust and stable expression of sGFP (S65T), the eGFP variant, which repeatedly had failed producing fluorescent signal in Laccaria in the past, was now easily obtained when translationally fused to a fungal intron-containing sequence, strongly suggests that the FP expression in Laccaria is indeed intron processing-dependent. This conclusion was later reconfirmed with cytosolic FP expression (see below).
Fluorescence microscopy of wild type, pHTB16202GFP and pHTB16202mCherry transformed Laccaria confirmed specific nuclear localization of H2B-FP. a Fluorescence signals detected in DAPI stained wild type (wt), pHTB16202mCherry (H2B-mCherry), and pHTB16202GFP (H2B-GFP) strains. b A magnification of (a) and shows DAPI staining of nuclei in the wild type fungus. c Specific nuclear localization in a selected pHTB16202mCherry strain. d Demonstrates specific nuclear localization in a selected pHTB16202GFP strain. a ×400 total magnification, rest of the panels ×1000 magnification. bf bright field
Expression of H2B-FP in Laccaria does not affect fungal growth. The growth of the wild-type fungus is visually comparable to the growth of pHTB16202-FP strains. a wt colonies on P5 medium after 2 weeks of growth. b Two pHTB16202mCherry transformant strains (the upper colonies on the plate) and two pHTB16202GFP transformants (the lower colonies on the plate) on P5 medium after 2 weeks of growth. c The same pHTB16202-FP transformants (and wt inoculated in the middle of the plate showing now growth) on P5 medium supplemented with 200 µg/ml hygromycin B after 2 weeks of growth. d, e Nuclear FP localization is stable in time irrespectively of the presence of antibiotic selection pressure. Fluorescence signals were clearly detectable in Laccaria hyphae both in pHTB16202mCherry (photo panel line d) and in pHTB16202GFP transformants (photo panel line e) after 1 month of growth in the absence of hygromycin B in the growth medium
A plasmid toolkit for flexible use of FP fusions in Laccaria and other homobasidiomycetes
We constructed a plasmid toolkit for “easy-to-clone” and technically flexible use of FP fusions in Laccaria and ECM studies, based on classic restriction enzyme (RE) and DNA ligase-based cloning of in-frame C-terminal fusions between genes of interest (GOI) and eGFP and mCherry markers. Vectors for both endogenous GOI promoter transcribed marker fusions, and fusions expressed from the constitutive heterologous Agaricus bisporus gpdII promoter, shown to be broadly recognized in homobasidiomycetes, were designed. Furthermore, these cloning vectors were specifically planned to work in concert with the ATMT binary vector pHg (Kemppainen and Pardo 2010) under hygromycin B selection.
As a result, cloning vectors pN-GFP and pN-mCherry were constructed for transcription from endogenous GOI promoters (Fig. 5a) and vectors pCEBN-GFP and pCEBN-mCherry (CEBN: Constitutive Expression in Basidiomycetes, N-terminal fusion of FP) for gpdII promoter expressed GOI-FP cloning (Fig. 4b). The basic technical features of the cloning vectors include: (1) SmaI (or its isoschizomer XmaI) cut-based in-frame fusion between the GOI and the FP genes via a five amino acids linker sequence. (2) Highly flexible multiple cloning sites, having up to 12 (pN-GFP and pN-mCherry) or up to 10 (pCEBN-GFP and pCEBN-mCherry) unique RE sites upstream of the fusion site, making introduction of the sequence of interest easy and practically independent of endogenous RE sites in it. (3) A unique SacI site for direct introduction of the fusion protein expression cassettes as full ampicillin-resistant plasmids into the pHg´s T-DNA. Due to apparent plasmid replication incompatibility, this full plasmid-cloning approach under double antibiotic selection in E. coli (simultaneous kanamycin and ampicillin selection) results predominantly in bacterial clones with predicted binary vector´s T-DNA structure (Fig. 6). Such dominant bacterial clones can be distinguished by their slightly weaker growth on solid growth medium, but at the same time, this precise T-DNA structure seems to result in amplification of the binary vector from the multicopy pBluescript replication origin, producing high plasmid prep yields (significantly higher than pHg alone). This notably facilitates final binary vector analysis and introduction to Agrobacterium. The full-plasmid introduction of FP fusion cassettes to pHg´s T-DNA also allows plasmid rescue under ampicillin resistance for studying the T-DNA integration sites in the fungal transformants (Kemppainen et al. 2008). Alternatively, in the case of endogenous SacI site in the GOI, the FP fusion expression cassettes can be excised as XbaI fragments and introduced to the unique XbaI site in pHg´s T-DNA. All of these cloning vectors also count with a rare-cutter NotI for their linearization. The use of the FP fusion vectors presented and completion of constructing the ATMT binary vector for fungal transformation requires one PCR reaction and two simple ligation steps (Fig. 6). Naturally, even though the finalized binary vectors are designed to be used in ATMT, they are also compatible with any other plasmid DNA-based fungal transformation methodology.
Schematic presentation of a pN-GFP and pN-mCherry plasmids designed for FP-fusion cloning of GOIs driven by endogenous promoters and b pCEBN-GFP and pCEBN-mCherry plasmids optimized for constitutive expression of GOI–FP fusions transcribed from A. bisporus gpdII promoter. All these cloning vectors contain highly flexible MCS for directional cloning purpose. The in-frame fusion between the GOI and FPs is generated via SmaI (or XmaI) site ligation and includes a 5 aa non-polar linker sequence
The pN-GFP, pN-mCherry, pCEBN-GFP, and pCEBN-mCherry cloning vectors are designed to work in concert with the binary vector pHg, which carries the hygromycin B resistance cassette. Constructing the final ATMT vector with the GOI-FP expression cassette requires in total one PCR and two simple ligation reactions. Once the GOI sequence is introduced into the cloning vectors, the FP-fusion expression cassettes can be liberated as full SacI linearized vector (or as XbaI excised cassettes) and cloned into the T-DNA of pHg. In the former case, double antibiotic pressure (kanamycin + ampicillin) is used for selecting the final ATMT transformation constructs. In the case of occurrence of both XbaI and SacI sites in the GOI, these FP fusion vectors can still be generated using rare-cutting restriction endonuclease NotI for vector linearization and cloning into the pHg
Evaluation of Laccaria Kozak sequence
As the in-frame cloning structures in pN-GFP and pN-mCherry were equivalent to the one used in endogenous histone H2B promoter-driven H2B-FP expression cassettes (vectors pHTB16202GFP and pHTB16202mCherry, described above) functionality of these plasmids needed no additional confirmation. However, the performance of pCEBN-GFP and pCEBN-mCherry required further evaluation. As in this case, the gene sequences without their native upstream regulatory sequences (i.e., 5´ UTR and the promoter sequence) were used, we first evaluated the Laccaria consensus Kozak sequence and its effect on transgene expression.
The Kozak sequence, which controls mRNA translation, varies across organisms (Nakagawa et al. 2008). For vertebrates, the consensus Kozak sequence (gcc)gccRccATGG, where R is a purine (A or G) (Kozak 1987, 2002) has been established, whereas TCA(C/a)(A/c)ATG(G/t)C (capital letters: conserved nucleotides with > 70% frequency, lower case letters alternative nucleotides with 50–70% frequency) was put forward for filamentous ascomycetes (Ballance 1986) and the adenine-rich sequence aAaAaAATGTCt (A at the critical position − 3) for Saccharomyces (Hamilton et al. 1987). A feature that appears conserved among ascomycetes is a strong preference for adenine, both at the position − 1 and − 3 (A of the ATG is considered the position + 1). In this context, the upstream sequence of HTB16202 (ACCATCTCTCATGGC; start codon underlined) showed minimal resemblance with the consensus Kozak sequences reported for ascomycete fungi. Especially, the lack of purine, but a cytosine, at the positions − 3, was unexpected. Therefore, we established the Laccaria consensus Kozak sequence based on 47 highly expressed genes of the fungus (Online Resources file 3 and 4). This analysis included 10 nt upstream and 2 nt downstream of the start codon and resulted in Laccaria consensus Kozak sequence as: NNCHNTCAHAATGGC (H = A, C or T, N = any, start codon underlined) (Fig. 7). Similarly to Kozak consensus sequences reported for other fungi, the strongly expressed genes in Laccaria have very high frequency of adenine (A) at the position − 3 (76.5% of analyzed genes), and adenine is also the preferred nucleotide at the position − 1 (40.0% of analyzed genes) and cytosine (C) at the position + 5 (42.5%). In addition, the preference for a guanine (G) at the position + 4 (48.9%) is a shared feature of the consensus Kozak sequences between Laccaria, filamentous ascomycetes, and vertebrates.
Laccaria consensus Kozak sequence (15 nucleotides analyzed from position − 10 to + 5) based on 47 highly transcribed gene models. The percentage of conserved nucleotides (≥ 40% frequency) at a given position is presented as superindex (N%); low-frequency nucleotides (≤ 10%) at a given position are presented as subindex in parenthesis N(x). Start codon ATG underlined, very strongly conserved − 3 adenine in bold
Especially, cytosine (C) at the position − 3 is rare in strongly expressed genes of Laccaria (present only in 3 out of 47 sequences analyzed. Concordantly, in Saccharomyces, a pyrimidine at position − 3 has been shown to lower protein production (Dvir et al. 2013). Therefore, the consensus versus non-consensus Kozak sequence, and especially, the effect of − 3 C versus − 3 A on transgene expression in Laccaria was studied further. Laccaria has three glyceraldehyde-3-phosphate dehydrogenases type I (GPD)-encoding genes, of which two are strongly expressed in free-living mycelium (JGIv2 ID #318873 and JGIv2 ID #295504, Online Resources file 3). Among these, gene 318873 has a Kozak sequence (AACTATCACAATGGT), which almost completely fulfills the Laccaria consensus Kozak sequence established (Fig. 7). We studied the performance of this sequence in comparison to the Laccaria pectin methyl esterase encoding gene LbPME1 (JGIv2 ID #245379), which has a native Kozak sequence with − 3 cytosine (TCTTTTCAAATGAA). To evaluate the effect of the 5´upstream sequences on transgene expression of LbPME1 in Laccaria, two transformant libraries were generated: one carrying an LbPME1 overexpression construct under control of A. bisporus gpdII promoter (the promoter sequence does not contain intact Kozak sequence) with the native Kozak of LbPME1 (− 3 C) and the other with Laccaria GPD gene “consensus respecting” Kozak (− 3 A) (Fig. 8a). Twelve randomly selected transformants from each transformant library were evaluated for LbPME1 gene expression levels by qRT-PCR (Fig. 8b). As expected, when the “consensus respecting” Kozak was applied the mean level of pectin methyl esterase transcripts was 3.4-fold higher than in the wild type, while the transformants generated with the non-consensus native gene Kozak showed only 1.5-fold mean target gene overexpression (Fig. 8b). Moreover, only two of the native Kozak strains had higher than twofold LbPME1 transcript levels as compared to wild type, while 10 out of 12 of the consensus Kozak transformants exceeded this value. In addition, the maximum overexpression level was higher in the latter strain set (7.4-fold with consensus versus 5.4-fold with native Kozak). These results clearly indicated that the presence of the consensus Kozak sequence has important effects on the transgene mRNA accumulation in Laccaria.
The effect of non-consensus and consensus Kozak sequences on transgene overexpression in Laccaria.a The pectin methyl esterase gene (LbPME1; JGIv2 ID#245379) was overexpressed under A. bisporus gpdII promoter. The transformation constructs carried the gene sequence of LbPME1 either with its native Kozak sequence (with a C at position − 3) or with the Laccaria glyceraldehyde 3-phosphate dehydrogenase gene (GPD) Kozak (JGIv2 ID #318873), which respects the characteristics of the consensus Kozak sequence established for the fungus. b Results from qRT-PCR analysis. LbPME expression (fold change) in 12 randomly selected OE transformant lines generated, with either native gene Kozak sequence or the Laccaria consensus Kozak sequence. Gene expression was normalized on histone H4 (JGIv2 ID #319764), ubiquitin (JGIv2 ID #446085), elongation factor 3 (JGIv2 ID #659644), and metalloprotease (JGIv2 ID #245379). Relative expression of the target gene was calculated against expression in wild-type Laccaria. Error bars represent ± SE. The transformant lines showing statistically significant target gene overexpression are marked with stars (t test, p < 0.05)
Confirmation of the plasmid toolkit functionality—constitutive promoter-driven H2B-FP nuclear localization and cytosolic FP expression in Laccaria
The cloning plasmids with constitutive A. bisporus gpdII promoter, pCEBN-GFP and pCEBN-mCherry, were then validated with nuclear FP localization and cytosolic expression of eGFP and mCherry in Laccaria. For nuclear FP expression, histone H2B (isoform HTB16202) was used once more as test gene, but now under control of the A. bisporus gdpII promoter. As the presence of the consensus Kozak sequence significantly improved transgene expression in Laccaria (Fig. 8), the cytosine at position − 3 in the native Kozak sequence of HTB16202 suggested possible suboptimal transgene mRNA accumulation and/or translation when transcribed from the heterologous promoter. Therefore, to confirm the functionality of the cloning vectors, these were evaluated with both the native HTB16202 Kozak and the “consensus respecting” Laccaria GPD gene Kozak sequence.
Four H2B–FP fusion constructs (pCEBN-GFP/KNH2B, pCEBN-GFP/KgpdH2B, pCEBN-mCherry/KNH2B, and pCEBN-mCherry/KgpdH2B, KN standing for native Kozak sequence and Kgpd for the GPD Kozak sequence) were used for ATMT of Laccaria (Fig. 9a). All vectors resulted in equivalent and very high transformation efficiencies (210–250%). Twelve randomly selected strains for each construct were evaluated for their nuclear FP expression. All four constructs, irrespective of the Kozak sequence used, produced clearly detectable and nuclear localized FP signal, confirming thus the functionality of the constitutive promoter-driven GOI-FP expression cassettes in these cloning vectors (Fig. 9b,c). The number of fungal lines showing nuclear fluorescence was slightly lower than what was produced with the endogenous promoter driven HTB16202–FP fusions, ranging from 58–67%, but similarly to the endogenous promoter driven constructs, the growth of the transformant did not appear affected by the constitutive H2B-FP expression. Equally, the most intense FP signals were detected in mitotic nuclei with condensed nuclear material (Fig. 9d). Applying different Kozak sequences did not result in apparent differences in H2B-FP expression. This can be related to the demonstrated very high stability of histone proteins in time (Bondy 1971) and their structural incorporation in nucleosomes, which could easily mask possible variations in transgene expression levels in the case of this particular fusion protein.
Functionality of pCEBN-GFP and pCEBN-mCherry as constitutive GOI–FP fusion protein expression vectors was tested with aLaccaria histone H2B gene, incorporating either its native non-consensus H2B Kozak or Laccaria GPD gene (JGIv2 ID #318873) consensus Kozak sequence in the expression cassettes. Efficient nuclear FP localization was obtained both for mCherry (b) and eGFP (c) with all four transformation constructs (The demonstrative photos shown were taken from transformant strains produced with consensus GPD Kozak sequence, ×400 magnification, scale bars 30 µm). d Close-up photos of Laccaria clamp connection and duplicated nuclei in a dikaryotic pCEBN-mCherry/KgpdH2B strain during cell division, ×1000 magnification, scale bars 10 µm
The pCEBN-GFP and pCEBN-mCherry vectors were further tested for launching cytosolic FP expression in Laccaria. As intron processing was previously shown to be required for successful FP expression in the fungus, native intron-containing sequences of a cytosolic Laccaria protein, together with the “consensus respecting” Kozak sequence, were cloned in-frame with the FPs in the vectors. These consisted of the 5′ fragment of Laccaria GPD gene (JGIv2.0 ID#318873) and 9 nt upstream sequence of its start codon. This 5′ GPD fragment contained the first two exons, introns and the beginning of the third exon of the gene. The resulting vectors pCEBN-cytosolGFP and pCEBN-cytosolmCherry (Fig. 10a) were used for ATMT of Laccaria. For confirming the intron processing-dependency of FP expression in Laccaria, we used intronless pCEBN-GFP and pCEBN-mCherry, with their MCS-modified (pCEBN-GFPmod and pCEBN-mCherrymod), to resemble the distance between the A. bisporus gpdII promoter and the GPD–FP fusions, as controls. All four vector constructs resulted in relative high transformation efficiencies (130–185%) and 12 randomly selected transformants were evaluated for cytosolic fluorescence. The results on cytosolic FP expression confirmed the central role of intron processing in efficient FP expression in Laccaria and the functionality of pCEBN-GFP and pCEBN-mCherry as GOI-FP cloning vectors. While none of the lines produced with the control vectors showed fluorescent signal, three and eight lines transformed with pCEBN-cytosolmCherry and pCEBN-cytosolGFP, respectively, had clearly fluorescent hyphae (Fig. 10b). In such FP-expressing lines, the fluorescent signal was present in all hyphae and appeared evenly distributed in the cytosol. The growth of the cytosolic FP expressing lines was not negatively affected (data not shown). The stability of cytosolic FP expression in Laccaria was further evaluated with two strongly and two moderately cytosolic eGFP and mCherry expressing lines, with and without antibiotic selection. Visual evaluation of FP signal after 2 weeks of growth under both conditions resulted in the conclusion that, while the cytosolic FP expression in Laccaria is well stable in time and clearly independent of hygromycin B in strong FP expressing lines, moderate FP expression is positively influenced by the antibiotic selection pressure. In these lines, the cytosolic fluorescence detected under hygromycin B growth was found stronger than when growing without it (data not shown). We postulate that this boosting effect on FP expression could be the result of stimulated, “forced” transcription of the hygromycin resistance-bearing marker gene under selective conditions, which consequently could result in more open chromatin structure at the whole transgene locus, facilitating thus as well transcription of the FP fusion cassettes.
Cytosolic FP expression in Laccaria is intron-processing dependent. Cytosolic FP expression cassettes (both for eGFP and mCherry) were constructed in pCEBN-FP cloning vectors incorporating 5′ fragment of Laccaria GPD gene (JGIv2 ID #318873) to create a pCEBN-cytosolFPs. b Both pCEBN-cytosolFP were functional in producing cytosolic fluorescence protein expression in the fungus. Photos of the wild-type mycelia shown as control, magnification ×400 (scale bars 30 µm), ×1000 magnification (scale bars 20 µm)
Discussion
Expression of fluorescent proteins (FP) fused with proteins naturally localizing to different cellular compartments offers a valuable tool for studying variable cell functions. In fungi, besides being expressed freely in the cytosol, FPs have been successfully targeted to different organelles such as nucleus, plasma membrane, peroxisomes, mitochondria, ER lumen, and Golgi (for reviews see Cormack 1998; Lorang et al. 2001; Bialecka-Fornal et al. 2016). Moreover, simultaneous expression of FPs with respectively different cellular localizations has made it possible to study functions of complex intracellular structures such as the hyphal tip apparatus (the Spitzenkörper) (Sudbery 2011). FPs are also highly exploited as in vivo markers in pathogenic plant–fungus interactions and their use has elucidated in multiple ways how these biotrophic fungi interact with their hosts. On the other hand, ectomycorrhizal (ECM) research had been limited by the lack of efficient FP expression in the asco- and basidiomycete fungi engaged in this symbiotic relation formed with tree roots. Even though some reports on successful FP expression in ECM fungi exist, such as GFP expression in the basidiomycete Hebeloma cylindrosporum (Müller et al. 2006; Rekangalt et al. 2007), DsRed2 in Suillus grevillei (Murata et al. 2006), and GFP expression in the ascomycete Tuber borchii (Brenna et al. 2014), these have not led to widespread use of FP markers in mycorrhizal research. Reasons for this can be speculated, but technical complications in genetic transformation of ECM fungi and presumably also low reproducibility in FP expression, due to complications in transgene expression in basidiomycetes in general, have most certainly contributed to the current situation. Moreover, while molecular tools for easy in-frame FP marker cloning are available for ascomycete studies (such as the plasmid sets introduced by Toews et al. 2004; Lange et al. 2014; Gong et al. 2015), FP-marker vector systems designed specifically for basidiomycetes are scarce and they are mostly only suited for promoter studies with cytosolic FP expression as a transcriptional reporter (Burns et al. 2005; Ford et al. 2016; Nishikawa et al. 2016).
We have studied the requirements for successful transgene expression, including intron processing and the effect of the Kozak sequence, in the model ECM basidiomycete Laccaria bicolor, and established both cytosolic and nuclear-targeted eGFP and mCherry expression. This is the first report on robust FP expression in Laccaria, currently the most important fungal model in ECM research. In addition, we present here an “easy-to-clone” plasmid toolkit for technically flexible use of these two FP markers. In comparison with other FP fusion/transformation vectors available for basidiomycetes, the vector set described here allows an easy cloning of translational C-terminal fusions between the gene of interest (GOI) and the FPs, and these protein fusions can be transcribed either from their endogenous regulatory sequences or from the constitutive A. bisporus gpdII promoter. Moreover, these in-frame cloning plasmids are designed to be directly compatible with Agrobacterium-mediated gene transfer (ATMT) under hygromycin B selection. Among the two FPs tested, mCherry undoubtedly performed superior to the assessed GFP variant. It produced more durable fluorescence signals and minimal hyphal autofluorescense was present at the detection wavelength range of this red FP.
We decided to use Laccaria H2B histone to target FP expression to the nucleus in hyphae. Two replication-dependent histone H2B isoforms, highly expressed in Laccaria, were identified in the genome of the fungus. The genes encoding them are localized in two fungal evolutionary conserved histone gene loci with their corresponding nucleosome histone H2A pairs. We had hypothesized that introns may be a requirement in the protein FP fusion for successful FP expression in Laccaria. Sequence analysis revealed the presence of introns in these Laccaria histone H2B genes, which is in line with the finding that filamentous asco- and basidiomycetes have intronic sequences in their replication-dependent histone genes (Woudt et al. 1983; Yun and Nishida 2011). The H2B isoform HTB16202 was used as an intron-containing sequence to target FPs to the nucleus. Our results have confirmed that incorporation of introns into the FP transcription cassettes is indispensable for efficient FP expression in Laccaria, a condition, which also explains previous unsuccessful attempts to launch FP expression in the fungus (Kemppainen et al. 2005). This is supported by reports showing that the presence or absence of introns has strong effects on mature mRNA or protein production in different eukaryotes. For example, expression of intronless transgenes, such as bacterial genes or eukaryotic cDNAs, is known to benefit from addition of intronic sequences (Callis et al. 1987). Inclusion of introns, especially in the 5´ UTR region of the transgenes, increases the amount of cellular mRNA and protein accumulation up to 100-fold (Brinster et al. 1988; Huang and Gorman 1990; Luehrsen and Walbot 1991; Bourdon et al. 2001; Lu and Cullen 2003; Samadder et al. 2008).
Interestingly, the intron dependence of FP expression varies significantly between fungal taxa and even between closely related species. While in ascomycetes, FP expression has been achieved with relative ease (Lorang et al. 2001), efficient transgene expression in many basidiomycetes has been less straightforward and especially dependent on intron processing. Intronless transgenes, such as FPs, are successfully transcribed, but no significant amount of protein is produced, resulting in failure of FP detection (Ma et al. 2001; Scholtmeijer et al. 2001; Godio et al. 2004). This difference between ascomycete and basidiomycete fungi, with respect to intron dependence of FP expression, is well illustrated by reports on fungal transformation with the binary vector pBGgHg (Chen et al. 2000), which carries a sGFP(S65T) expression cassette lacking introns. Similar to Laccaria, failures in GFP expression have been reported, for example, for pBGgHg-transformed basidiomycetes Agaricus bisporus, Hyphaloma sublateritium, and Armillaria mellea (Chen et al. 2000; Godio et al. 2004; Baumgartner et al. 2010). On the other hand, filamentous ascomycete fungi, such as Monascus purpureus and Fusarium oxysporum f.sp. gladioli carrying the same transgenic constructs in their genome, show strong GFP signals (Campoy et al. 2003; Lakshman et al. 2012). In addition, this intron dependence of FP expression varies, not only between basidio- and ascomycetes, but also between different basidiomycete species. Similar to Laccaria, successful FP expression required incorporation of intronic sequences for example in Schizophyllum commune, Phanerochaete chrysosporium, Coprinus cinerea, and Suillus gravillei, (Lugones et al. 1999; Ma et al. 2001; Burns et al. 2005; Murata et al. 2006). On the other hand, for instance in Ustilago maydis, Cryptococcus neoformans, Pisolithus tinctorius, and H. cylindrosporum, FP expression has been succesfully established with transgenic constructs lacking introns (Spellig et al. 1996; del Poeta et al. 1999; Rodríguez-Tovar et al. 2005; Müller et al. 2006).
While the reasons for the species dependence of intron requirement for FP expression in basidiomycetes remain unclear, the eukaryotic cellular mechanisms behind the “intron effect” are widely studied. Introns are shown to both initiate and positively affect gene expression by a mechanism called intron-mediated enhancement (IME) (for reviews, see Gallegos and Rose 2015; Laxa 2017). IME is reported to happen in all eukaryotic kingdoms, including fungi (Callis et al. 1987; Okkema et al. 1993; Furger et al. 2002; Moabbi et al. 2012; Jiang et al. 2015) and it appears to be a complex outcome of multiple components. These include interactions between the splicing machinery and other factors involved in mRNA synthesis and maturation. Moreover, IME influences not only the mRNA production, stability, and nuclear export, but also translation efficiency (Le Hir et al. 2001; Maniatis and Reed 2002; Wiegand et al. 2003; Curi et al. 2005; Dahan et al. 2011). Clearly, more research is needed to understand the mechanisms behind IME, and interestingly, intron splicing is not an absolute requirement for IME effects either. Some introns are reported to increase mRNA accumulation in the absence of their splicing (Rose and Beliakoff 2000) and recent experimental data suggest that the presence of intronic sequences in a gene affects directly transcription initiation, possibly by increasing the access of the transcription machinery to the DNA (Gallegos and Rose 2017). In addition, studies on IME in Saccharomyces propose that the transcription stimulation effect of introns in this fungus depends on DNA looping that brings the 5ʹ and 3ʹ ends of a gene into proximity, a condition which would favor transcription re-initiation (Moabbi et al. 2012).
Even though all cellular mechanisms influencing IMT remain to be more thoroughly studied, the effect of IMT on transgene expression is, however, indisputable, also in Laccaria. This was well demonstrated by comparison of intron-containing and intronless expression cassettes for launching cytosolic FP expression. While none of the evaluated fungal lines transformed with an intronless construct showed detectable fluorescence, an important percentage of the lines transformed with the intron-containing FP cassette expressed the FP proteins. Therefore, based on our results on intron dependence of transgene expression in Laccaria, we strongly recommend that genomic gene sequences, instead of cDNAs, should be utilized in FP fusion and gene overexpression studies with this fungus in future.
Naturally, not only transcription but also translation efficiency affects gene expression. Concordantly, quantitative proteomic data show that protein and mRNA concentrations are only moderately correlated (Bantscheff et al. 2012; Bensimon et al. 2012) and the regulation of mRNA translation efficiency is highly central to control of gene expression in eukaryotes (Schwanhäusser et al. 2011). While lack of introns can directly affect transgene translation via IME pathways, reduced translation initiation has equally been shown to strongly promote mRNA degradation, making translation initiation an important regulator of mRNA stability as well (Huch and Nissan 2014). Therefore, suboptimal translation can have important implications, both directly, and via reduced transgene transcript stability, on protein accumulation, and in the case of the FP expression result in poor fluorescent signals in the cells. In eukaryotes, translation initiation, which involves start codon recognition by ribosomes via a mechanisms called ribosome scanning (for a review, see Hinnebusch 2017) is strongly dependent on the Kozak sequence, a sequence motif in the 5′UTR of mRNAs, which overlaps with the translation start codon itself. As the Kozak sequence varies to some extent in eukaryotes, the consensus Kozak established in one organism can be a weak predictor of translation efficiency in another one. Generally, no special attention has been paid on incorporation of Kozak sequences into the transgene expression cassettes in basidiomycete studies, and both the specific Kozak consensus sequence features and its effect on translation efficiency in filamentous basidiomycetes are currently very poorly investigated. We have established the consensus Kozak sequence for Laccaria (NNCHNTCAHAATGGC) based on a set of highly expressed Laccaria genes. The general characteristics of the Kozak sequence in Laccaria respect what was previously reported for yeast and filamentous ascomycetes (Ballance 1986; Hamilton et al. 1987) and especially the − 3A and − 1A appear highly conserved features of fungal Kozak sequences. Most importantly, evaluation of the mRNA levels produced from a constitutively expressed transgene cassette containing either a “non-consensus” respecting Kozak or Laccaria consensus-type Kozak sequence revealed a significant “boosting effect” of the consensus sequence on transgene mRNA accumulation. These results highlight not only the importance of intron-incorporation but also the use of an optimal Kozak sequence in transgenic studies in Laccaria. The detected positive effect of the consensus-type Kozak sequence is most probably linked to the tight connection between mRNA stability and efficient transcript translation, especially translation initiation, shown both in pro- and eukaryotes (for reviews see Radhakrishnan and Green 2016; Bicknell and Ricci 2017). Naturally, high cellular transcript levels cannot be directly extrapolated to high translation efficiency. However, we suggest that the Laccaria consensus Kozak sequence established here is a close estimate for an optimal 5ʹ UTR sequence motif to be applied to future transgenic studies, and that this technical aspect should not be overlooked.
In the light of sequence characteristics established for the consensus Kozak in Laccaria, the 5´UTR of strongly expressed Laccaria histone H2B isoform HTB16202 is intriguing. The HTB16202 has a non-consensus Kozak sequence, with rare − 3C, which suggests inefficient translation of its transcript. However, both the H2B-FP construct transcribed from the endogenous histone promoter, and the one from the constitutive heterologous promoter with the native Kozak sequence, resulted in strong nuclear FP labeling. This demonstrates that, despite the non-optimal Kozak, some other factors were sufficient for efficient translation of this gene. Recently, a novel gene expression regulation pathway for eukaryotes was revealed in Saccharomyces. In yeast, the ribosomes containing the ribosomal protein 26 (Rps26) have been shown to recognize the consensus Kozak sequence and are, consequently, responsible of efficiently translating these gene transcripts. On the other hand, Rps26-depleted ribosomes translate mRNAs from selected stress-response pathways, which show low Kozak sequence conservation. The relative abundance of these precise ribosomes increases under stress conditions, which leads to increased translation of their target mRNAs (Ferretti et al. 2017; Dalla Venezia et al. 2019). In the light of this novel information on ribosome scanning in Saccharomyces, together with the 5ʹ upstream sequence characteristics of histone H2B isoform HTB16202, it is possible that the translation of this gene is regulated by the cell cycle or other stimuli via a different ribosome populations in Laccaria. In addition, histone proteins are highly stable in time with an estimated half-life of 19 days for histone proteins in chicken brain tissue (Bondy 1971). As histone FPs are also demonstrated to get incorporated into nucleosomes in vivo, (Kanda et al. 1998), similar increased stability can be proposed for H2B–FP fusion protein in Laccaria. Therefore, in the presence of high transcript levels, even sub-optimal translation can theoretically be expected to generate long-lasting nuclear fluorescent signals. Also, the local cellular concentration of nuclear-directed FP is high, facilitating thus detection of the FP signal, even under inefficient translation conditions. However, the situation might be different when a weak or inducible, and especially not the native promoter is applied, together with a sub-optimal native gene Kozak sequence, and particularly in combination with a cytosolic GOI-FP reporter. In concordance with this, not only the Kozak sequence itself, but also the nucleotides upstream of it have recently been demonstrated to strongly influence gene expression in S. cerevisiae (Li et al. 2017). Therefore, when the plasmid system described here is to be used for GOI-FP expression from other than the native GOI promoter, and when the native Kozak sequence of the GOI differs especially at the nucleotide position − 3 from the consensus established in Laccaria, we recommend that the consensus-type Kozak, such as the one of Laccaria GPD gene, is applied for transgene expression. This is to assure maximum transgene translational strength, which would consequently most likely reflect an increased in FP-marker mRNA stability.
Today, the role of a majority of Laccaria genes, postulated important for the ECM interaction, is supported solely by bioinformatic analyses and transcript profiles. Therefore, further functional studies are urgently needed to confirm the true participation of the symbiosis-regulated genes and to demonstrate the exact cellular actions of their protein products in the establishment and function of ECM. Knowledge on basic requirements of efficient transgene expression in Laccaria, together with robust FP expression and the cloning/transformation vector-system described here make now possible in vivo protein localization and promoter activity studies in ECM research. Naturally, the molecular tools described here can also be exploited in studies of other biotrophic or saprotrophic basidiomycete species susceptible to genetic transformation.
Conclusions
We have created transgenic dikaryotic Laccaria lines with strong nuclear-localized and cytosolic GFP and mCherry expression, and these are available for studies to address hyphal behavior in ECM interaction. We have proven that the presence of introns is necessary for efficient FP expression and that the use of the consensus Kozak sequence significantly increases transcript accumulation of the transgenes in the fungus. Fluorescent protein expression in Laccaria opens novel possibilities for both ECM research and homobasidiomycete studies in general, and we expect that the FP marker tools and findings presented here on requirements of transgene expression in Laccaria, will rapidly shed green and red light on various aspects of ectomycorrhizal plant–fungal interactions.
References
Arvidsson S, Kwasniewski M, Riaño-Pachón DM, Mueller-Roeber B (2008) QuantPrime -a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinform 9:465
Ballance DJ (1986) Sequences important for gene expression in filamentous fungi. Yeast 2(4):229–236
Bantscheff M, Lemeer S, Savitski MM, Kuster B (2012) Quantitative mass spectrometry in proteomics: critical review update from 2007 to the present. Anal Bioanal Chem 404(4):939–965
Baumgartner K, Fujiyoshi P, Foster GD, Bailey AM (2010) Agrobacterium tumefaciens-mediated transformation for investigation of somatic recombination in the fungal pathogen Armillaria mellea. Appl Environ Microbiol 76(24):7990–7996
Bensimon A, Heck AJ, Aebersold R (2012) Mass spectrometry-based proteomics and network biology. Annu Rev Biochem 81:379–405
Bialecka-Fornal M, Makushok T, Rafelski SM (2016) A review of fluorescent proteins for use in yeast. Methods Mol Biol 1369:309–346
Bicknell AA, Ricci EP (2017) When mRNA translation meets decay. Biochem Soc Trans 45(2):339–351
Bielska E, Higuchi Y, Schuster M, Steinberg N, Kilaru S, Talbot NJ, Steinberg G (2014) Long-distance endosome trafficking drives fungal effector production during plant infection. Nat Commun 5:5097
Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, Dumas C, Haseloff J, Berger F (2001) Dynamic analyses of the expression of the HISTONE:YFP fusion protein in arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13(3):495–509
Bondy SC (1971) The synthesis and decay of histone fractions and of deoxyribonucleic acid in the developing avian brain. Biochem J 123(3):465–469
Bourdon V, Harvey A, Lonsdale DM (2001) Introns and their positions affect the translational activity of mRNA in plant cells. EMBO Rep 2(5):394–398
Brenna A, Montanini B, Muggiano E, Proietto M, Filetici P, Ottonello S, Ballario P (2014) Integrative gene transfer in the truffle Tuber borchii by Agrobacterium tumefaciens-mediated transformation. AMB Express 4:43
Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD (1988) Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA 85(3):836–840
Burns C, Gregory KE, Kirby M, Cheung MK, Riquelme M, Elliott TJ, Challen MP, Bailey A, Foster GD (2005) Efficient GFP expression in the mushrooms Agaricus bisporus and Coprinus cinereus requires introns. Fungal Genet Biol 42(3):191–199
Callis J, Fromm M, Walbot V (1987) Introns increase gene expression in cultured maize cells. Genes Dev 1(10):1183–1200
Campoy S, Pérez F, Martín JF, Gutiérrez S, Liras P (2003) Stable transformants of the azaphilone pigment-producing Monascus purpureus obtained by protoplast transformation and Agrobacterium-mediated DNA transfer. Curr Genet 43(6):447–452
Chen X, Stone M, Schlagnhaufer C, Romaine CP (2000) A fruiting body tissue method for efficient Agrobacterium-mediated transformation of Agaricus bisporus. Appl Environ Microbiol 66(10):4510–4513
Chiu W-L, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6(3):325–330
Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA (2010) Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev 90(3):1103–1163
Cormack B (1998) Green fluorescent protein as a reporter of transcription and protein localization in fungi. Curr Opin Microbiol 1(4):406–410
Cormack BP, Bertram G, Egerton M, Gow NA, Falkow S, Brown AJ (1997) Yeast-enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans. Microbiology 143(Pt 2):303–311
Curi GC, Chan RL, Gonzalez DH (2005) The leader intron of Arabidopsis thaliana genes encoding cytochrome c oxidase subunit 5c promotes high-level expression by increasing transcript abundance and translation efficiency. J Exp Bot 56(419):2563–2571
Dagdas YF, Yoshino K, Dagdas G, Ryder LS, Bielska E, Steinberg G, Talbot NJ (2012) Septin-mediated plant cell invasion by the rice blast fungus Magnaporthe oryzae. Science 336(6088):1590–1595
Dahan O, Gingold H, Pilpel Y (2011) Regulatory mechanisms and networks couple the different phases of gene expression. Trends Genet 27(8):316–322
Dalla Venezia N, Vincent A, Marcel V, Catez F, Diaz JJ (2019) Emerging role of eukaryote ribosomes in translational control. Int J Mol Sci 20(5):E1226
del Poeta M, Toffaletti DL, Rude TH, Sparks SD, Heitman J, Perfect JR (1999) Cryptococcus neoformans differential gene expression detected in vitro and in vivo with green fluorescent protein. Infect Immun 67(4):1812–1920
Di Battista C, Selosse MA, Bouchard D, Stenström E, Le Tacon F (1996) Variation in symbiotic efficiency, phenotypic characters and ploidy level among different isolates of the ectomycorrhizal basidiomycete Laccaria bicolor strain S238. Mycol Res 100:1315–1324
Ding DQ, Matsuda A, Okamasa K, Nagahama Y, Haraguchi T, Hiraoka Y (2016) Meiotic cohesin-based chromosome structure is essential for homologous chromosome pairing in Schizosaccharomyces pombe. Chromosoma 125(2):205–214
Doehlemann G, van der Linde K, Assmann D, Schwammbach D, Hof A, Mohanty A, Jackson D, Kahmann R (2009) Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog 5(2):e1000290
Dvir S, Velten L, Sharon E, Zeevi D, Carey LB, Weinberger A, Segal E (2013) Deciphering the rules by which 5′-UTR sequences affect protein expression in yeast. Proc Natl Acad Sci 110(30):E2792–E2801
Ferretti MB, Ghalei H, Ward EA, Potts EL, Karbstein K (2017) Rps26 directs mRNA-specific translation by recognition of Kozak sequence elements. Nat Struct Mol Biol 24(9):700–707
Ford KL, Baumgartner K, Henricot B, Bailey AM, Foster GD (2016) A native promoter and inclusion of an intron is necessary for efficient expression of GFP or mRFP in Armillaria mellea. Sci Rep 6:29226
Fraser ST, Hadjantonakis AK, Sahr KE, Willey S, Kelly OG, Jones EA, Dickinson ME, Baron MH (2005) Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis 42(3):162–171
Furger A, O'Sullivan JM, Binnie A, Lee BA, Proudfoot NJ (2002) Promoter proximal splice sites enhance transcription. Genes Dev 16(21):2792–2799
Gallegos JE, Rose AB (2015) The enduring mystery of intron-mediated enhancement. Plant Sci 237:8–15
Gallegos JE, Rose AB (2017) Intron DNA sequences can be more important than the proximal promoter in determining the site of transcript initiation. Plant Cell 29(4):843–853
Godio RP, Fouces R, Gudiña EJ, Martín JF (2004) Agrobacterium tumefaciens-mediated transformation of the antitumor clavaric acid-producing basidiomycete Hypholoma sublateritium. Curr Genet 46(5):287–294
Gong X, Hurtado O, Wang B, Wu C, Yi M, Giraldo M, Valent B, Goodin M, Farman M (2015) pFPL vectors for high-throughput protein localization in fungi: detecting cytoplasmic accumulation of putative effector proteins. Mol Plant Microbe Interact 28(2):107–121
Grzegorski SJ, Chiari EF, Robbins A, Kish PE, Kahana A (2014) Natural variability of Kozak sequences correlates with function in a zebrafish model. PLoS ONE 9(9):e108475
Hadjantonakis AK, Papaioannou VE (2004) Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol 4:33
Hamilton R, Watanabe CK, De Boer HA (1987) Compilation and comparison of the sequence context around the AUG start codons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res 15:3581–3593
Haruyama N, Cho A, Kulkarni AB (2009) Overview: engineering transgenic constructs and mice. Curr Protoc Cell Biol 42(1):19.10.1–19.10.9
Hinnebusch AG (2017) Structural insights into the mechanism of scanning and start codon recognition in eukaryotic translation initiation. Trends Biochem Sci 42(8):589–611
Huang MT, Gorman CM (1990) Intervening sequences increase efficiency of RNA 3' processing and accumulation of cytoplasmic RNA. Nucleic Acids Res 18(4):937–947
Huch S, Nissan T (2014) Interrelations between translation and general mRNA degradation in yeast. Wiley Interdiscip Rev RNA 5(6):747–763
Jackson RJ, Hellen CU, Pestova TV (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11:113–127
Jiang D, Berger F (2017) Histone variants in plant transcriptional regulation. Biochim Biophys Acta Gene Regul Mech 1860(1):123–130
Jiang L, Huang C, Sun Q, Guo H, Cheng T, Peng Z, Dang Y, Liu W, Xu G, Xia Q (2015) The 5'-UTR intron of the midgut-specific BmAPN4 gene affects the level and location of expression in transgenic silkworms. Insect Biochem Mol Biol 63:1–6
Kanda T, Sullivan KF, Wahl GM (1998) Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol 8(7):377–385
Kemppainen M, Pardo A (2010) pHg/pSILBAγ vector system for efficient gene silencing in homobasidiomycetes: optimization of ihpRNA—triggering in the mycorrhizal fungus Laccaria bicolor. Microb Biotechnol 3:178–200
Kemppainen M, Circosta A, Tagu D, Martin F, Pardo AG (2005) Agrobacterium-mediated transformation of the ectomycorrhizal symbiont Laccaria bicolor S238N. Mycorrhiza 16(1):19–22
Kemppainen M, Duplessis S, Martin F, Pardo AG (2008) T-DNA insertion, plasmid rescue and integration analysis in the model mycorrhizal fungus Laccaria bicolor. Microb Biotechnol 1:258–269
Kemppainen M, Duplessis S, Martin F, Pardo AG (2009) RNA silencing in the model mycorrhizal fungus Laccaria bicolor: gene knock-down of nitrate reductase results in inhibition of symbiosis with Populus. Environ Microbiol 11:1878–1896
Khang CH, Berruyer R, Giraldo MC, Kankanala P, Park SY, Czymmek K, Kang S, Valent B (2010) Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22(4):1388–1403
Kilaru S, Schuster M, Studholme D, Soanes D, Lin C, Talbot NJ, Steinberg G (2015) A codon-optimized green fluorescent protein for live cell imaging in Zymoseptoria tritici. Fungal Genet Biol 79:125–131
Kozak M (1984) Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature 308(5956):241–246
Kozak M (1987) At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 196:947–950
Kozak M (2002) Pushing the limits of the scanning mechanism for initiation of translation. Gene 299(1–2):1–34
Lakshman D, Pandey R, Kamo K, Bauchan G, Mitra A (2012) Genetic transformation of Fusarium oxysporum f.sp. gladioli with Agrobacterium to study pathogenesis in Gladiolus. Eur J Plant Pathol 133(3):729–738
Lange M, Oliveira-Garcia E, Deising HB, Peiter E (2014) A modular plasmid system for protein co-localization and bimolecular fluorescence complementation in filamentous fungi. Curr Genet 60(4):343–350
Lanver D, Tollot M, Schweizer G, Lo Presti L, Reissmann S, Ma LS, Schuster M, Tanaka S, Liang L, Ludwig N, Kahmann R (2017) Ustilago maydis effectors and their impact on virulence. Nat Rev Microbiol 15(7):409–421
Laxa M (2017) Intron-mediated enhancement: a tool for heterologous gene expression in plants? Front Plant Sci 7:1977
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology 9:963–967
Le Hir H, Gatfield D, Izaurralde E, Moore MJ (2001) The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J 20(17):4987–4997
Le Hir H, Nott A, Moore MJ (2003) How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci 28(4):215–220
Leroch M, Mernke D, Koppenhoefer D, Schneider P, Mosbach A, Doehlemann G, Hahn M (2011) Living colors in the gray mold pathogen Botrytis cinerea: codon-optimized genes encoding green fluorescent protein and mCherry, which exhibit bright fluorescence. Appl Environ Microbiol 77(9):2887–2897
Li J, Liang Q, Song W, Marchisio MA (2017) Nucleotides upstream of the Kozak sequence strongly influence gene expression in the yeast S. cerevisiae. J Biol Eng 11:25
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Lorang JM, Tuori RP, Martinez JP, Sawyer TL, Redman RS, Rollins JA, Wolpert TJ, Johnson KB, Rodriguez RJ, Dickman MB, Ciuffetti LM (2001) Green fluorescent protein is lighting up fungal biology. Appl Environ Microbiol 67(5):1987–1994
Lu S, Cullen BR (2003) Analysis of the stimulatory effect of splicing on mRNA production and utilization in mammalian cells. RNA 9(5):618–630
Luehrsen KR, Walbot V (1991) Intron enhancement of gene expression and the splicing efficiency of introns in maize cells. Mol Gen Genet 225(1):81–93
Luger K (2006) Dynamic nucleosomes. Chromosome Res 14(1):5–16
Lugones LG, Scholtmeijer K, Klootwijk R, Wessels JG (1999) Introns are necessary for mRNA accumulation in Schizophyllum commune. Mol Microbiol 32(4):681–689
Ma B, Mayfield MB, Gold MH (2001) The green fluorescent protein gene functions as a reporter of gene expression in Phanerochaete chrysosporium. Appl Environ Microbiol 67:948–955
Maniatis T, Reed R (2002) An extensive network of coupling among gene expression machines. Nature 416(6880):499–506
Martin F, Aerts A, Ahrén D, Brun A, Danchin EG, Duchaussoy F, Gibon J, Kohler A et al (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452(7183):88–92
Martin F, Kohler A, Murat C, Veneault-Fourrey C, Hibbett DS (2016) Unearthing the roots of ectomycorrhizal symbioses. Nat Rev Microbiol 14(12):760–773
Maruyama J, Nakajima H, Kitamoto K (2001) Visualization of nuclei in Aspergillus oryzae with EGFP and analysis of the number of nuclei in each conidium by FACS. Biosci Biotechnol Biochem 65(7):1504–1510
Moabbi AM, Agarwal N, El Kaderi B, Ansari A (2012) Role for gene looping in intron-mediated enhancement of transcription. Proc Natl Acad Sci USA 109(22):8505–8510
Molden RC, Bhanu NV, LeRoy G, Arnaudo AM, Garcia BA (2015) Multi-faceted quantitative proteomics analysis of histone H2B isoforms and their modifications. Epigenet Chromatin 8:15
Müller T, Benjdia M, Avolio M, Voigt B, Menzel D, Pardo A, Frommer WB, Wipf D (2006) Functional expression of the green fluorescent protein in the ectomycorrhizal model fungus Hebeloma cylindrosporum. Mycorrhiza 16(6):437–442
Murata H, Sunagawa M, Yamazaki T, Shishido K, Igasaki T (2006) Expression of the autofluorescent protein, DsRed2, in the recombinants of the ectomycorrhizal basidiomycete, Suillus grevillei, generated by Agrobacterium-mediated transformation. Mycorrhiza 16(6):407–412
Nakagawa S, Niimura Y, Gojobori T, Tanaka H, Miura K (2008) Diversity of preferred nucleotide sequences around the translation initiation codon in eukaryote genomes. Nucleic Acids Res 36(3):861–871
Nishikawa R, Yoshida M, Noda T, Okuhara T, Taguchi G, Inatomi S, Shimosaka M (2016) pFungiway: a series of plasmid vectors used for gene manipulation in fungi. Ann Microbiol 66(2):825–832
Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A (1993) Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135(2):385–404
Plett J, Kemppainen MJ, Kale S, Kohler A, Legue V, Brun A, Tyler BM, Pardo AG, Martin F (2011) A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr Biol 21:1197–1203
Plett JM, Daguerre Y, Wittulsky S, Vayssières A, Deveau A, Melton SJ, Kohler A, Morrell-Falvey JL, Brun A, Veneault-Fourrey C, Martin F (2014) Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proc Natl Acad Sci USA 111(22):8299–8304
Plett JM, Tisserant E, Brun A, Morin E, Grigoriev IV, Kuo A, Martin F, Kohler A (2015) The mutualist Laccaria bicolor expresses a core gene regulon during the colonization of diverse host plants and a variable regulon to counteract host-specific defenses. Mol Plant Microbe Interact 28(3):261–273
Plett JM, Yin H, Mewalal R, Hu R, Li T, Ranjan P, Jawdy S, De Paoli HC, Butler G, Burch-Smith TM, Guo HB, Ju Chen C, Kohler A, Anderson IC, Labbé JL, Martin F, Tuskan GA, Yang X (2017) Populus trichocarpa encodes small, effector-like secreted proteins that are highly induced during mutualistic symbiosis. Sci Rep 7(1):382
Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ (1992) Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111(2):229–233
Radhakrishnan A, Green R (2016) Connections underlying translation and mRNA stability. J Mol Biol 428(18):3558–3564
Rech C, Engh I, Kück U (2007) Detection of hyphal fusion in filamentous fungi using differently fluorescence-labeled histones. Curr Genet 52(5–6):259–266
Rekangalt D, Verner MC, Kües U, Walser PJ, Marmeisse R, Debaud JC, Fraissinet-Tachet L (2007) Green fluorescent protein expression in the symbiotic basidiomycete fungus Hebeloma cylindrosporum. FEMS Microbiol Lett 268(1):67–72
Rodríguez-Tovar AV, Ruiz-Medrano R, Herrera-Martínez A, Barrera-Figueroa BE, Hidalgo-Lara ME, Reyes-Márquez BE, Cabrera-Ponce JL, Valdés M, Xoconostle-Cázares B (2005) Stable genetic transformation of the ectomycorrhizal fungus Pisolithus tinctorius. J Microbiol Methods 63(1):45–54
Rosa S, Ntoukakis V, Ohmido N, Pendle A, Abranches R, Shaw P (2014) Cell differentiation and development in Arabidopsis are associated with changes in histone dynamics at the single-cell level. Plant Cell 26(12):4821–4833
Rose AB, Beliakoff JA (2000) Intron-mediated enhancement of gene expression independent of unique intron sequences and splicing. Plant Physiol 122(2):535–542
Samadder P, Sivamani E, Lu J, Li X, Qu R (2008) Transcriptional and post-transcriptional enhancement of gene expression by the 5' UTR intron of rice rubi3 gene in transgenic rice cells. Mol Genet Genomics 279(4):429–439
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Schoberle TJ, Nguyen-Coleman CK, May GS (2013) Plasmids for increased efficiency of vector construction and genetic engineering in filamentous fungi. Fungal Genet Biol 58–59:1–9
Scholtmeijer K, Wösten HA, Springer J, Wessels JG (2001) Effect of introns and AT-rich sequences on expression of the bacterial hygromycin B resistance gene in the basidiomycete Schizophyllum commune. Appl Environ Microbiol 67(1):481–483
Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22(12):1567–1572
Shipman EN, Jones K, Jenkinson CB, Kim DW, Zhu J, Khang CH (2017) Nuclear and structural dynamics during the establishment of a specialized effector-secreting cell by Magnaporthe oryzae in living rice cells. BMC Cell Biol 18(1):11
Smith S, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, London
Spellig T, Bottin A, Kahmann R (1996) Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis. Mol Gen Genet 252(5):503–509
Sudbery P (2011) Fluorescent proteins illuminate the structure and function of the hyphal tip apparatus. Fungal Genet Biol 48(9):849–857
Toews MW, Warmbold J, Konzack S, Rischitor P, Veith D, Vienken K, Vinuesa C, Wei H, Fischer R (2004) Establishment of mRFP1 as a fluorescent marker in Aspergillus nidulans and construction of expression vectors for high-throughput protein tagging using recombination in vitro (GATEWAY). Curr Genet 45(6):383–389
Vanden Wymelenberg AJ, Cullen D, Spear RN, Schoenike B, Andrews JH (1997) Expression of green fluorescent protein in Aureobasidium pullulans and quantification of the fungus on leaf surfaces. Biotechniques 23(4):686–690
Vayssières A, Pěnčík A, Felten J, Kohler A, Ljung K, Martin F, Legué V (2015) Development of the Poplar-Laccaria bicolor Ectomycorrhiza modifies root auxin metabolism, signaling, and response. Plant Physiol 169(1):890–902
Walter D, Matter A, Fahrenkrog B (2010) Bre1p-mediated histone H2B ubiquitylation regulates apoptosis in Saccharomyces cerevisiae. J Cell Sci 123(Pt 11):1931–1939
Wiegand HL, Lu S, Cullen BR (2003) Exon junction complexes mediate the enhancing effect of splicing on mRNA expression. Proc Natl Acad Sci USA 100(20):11327–11332
Woudt LP, Pastink A, Kempers-Veenstra AE, Jansen AE, Mager WH, Planta RJ (1983) The genes coding for histone H3 and H4 in Neurospora crassa are unique and contain intervening sequences. Nucleic Acids Res 11(16):5347–5360
Yun CS, Nishida H (2011) Distribution of introns in fungal histone genes. PLoS ONE 6(1):e16548
Acknowledgements
Funding to M.K. and A.P. was provided by grants from National University of Quilmes, National Council of Scientific and Technical Research (CONICET), and National Agency for Scientific and Technological Promotion (ANPCyT), Argentina. J.C. was funded by the Kempe foundations grant (SMK15-33) and The Swedish Research Council FORMAS (942-2015-539). We thank L. Nilsson for the help with media preparations during experiments. Nucleotide sequence data reported are available in the GenBank databases under the accession numbers MN781138, MN781139, MN781140 and MN781141.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
294_2020_1060_MOESM1_ESM.pdf
Supplementary file1 Online Resource file 1 (Table S1) Primers used in this study for constructing the cloning vectors presented and the GOI-FP fusion constructs used in Laccaria ATMT. (Table S2) Primers used in quantitative RT-PCR analyses. HK: housekeeping gene used for normalization (PDF 303 kb)
294_2020_1060_MOESM2_ESM.pdf
Supplementary file2 Online Resource file 2 pN-GFP, pN-mCherry, pCEBN-GFP and pCEBN-mCherry cloning vector sequences (between M13F and M13R primer binding sites) (PDF 514 kb)
294_2020_1060_MOESM3_ESM.pdf
Supplementary file3 Online Resource file 3 Laccaria and Ustilago codon usage. Nucleotide and protein sequence analyses involving Laccaria histone H2B isoforms. Laccaria consensus Kozak sequence analysis (PDF 1182 kb)
294_2020_1060_MOESM4_ESM.xlsx
Supplementary file4 Online Resource file 4 Excel file containing information of Laccaria gene models used for establishing the consensus Kozak sequence (XLSX 21 kb)
Rights and permissions
About this article
Cite this article
Kemppainen, M., Chowdhury, J., Lundberg-Felten, J. et al. Fluorescent protein expression in the ectomycorrhizal fungus Laccaria bicolor: a plasmid toolkit for easy use of fluorescent markers in basidiomycetes. Curr Genet 66, 791–811 (2020). https://doi.org/10.1007/s00294-020-01060-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-020-01060-4