Abstract
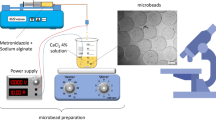
The aim of this study was the optimization of the gallic acid (GA) encapsulation efficiency within calcium alginate microparticles by the ionotropic gelation technique, using Box-Behnken design for the surface methodology response. For this purpose, three independent variables were selected: sodium alginate concentration (X1), calcium chloride concentration (X2), and gallic acid concentrations (X3). The influence of each variable on the encapsulation efficiency was evaluated. The optimum conditions to reach maximum encapsulation efficiency were found to be: X1 = 30 g/l (3%, w/v), X2 = 21.63 g/l (2.163%, w/v) and X3 = 15 g/l (1.5%, w/v), respectively. The encapsulation efficiency was determined to be 42.8%. The obtained microbeads were further examined using differential scanning calorimetry (DSC) and Fourier transform infrared (ATR-FTIR), and the inclusion of gallic acid was confirmed. The gallic acid concentration (X3) is the statistically significant factor in the optimization process. In addition, no autoxidation of the gallic acid compound was observed in the formulated calcium alginate microbeads. Scanning electron microscope (SEM) analysis showed that the shape of the particle was spherical for all formulations and their surface is wrinkled. The release study of the gallic acid carried out in an aqueous medium at pH value 6.8, showed that the GA release pattern was fast for all systems studied (85% at 20 min), and the profile of the release was influenced by the size of the calcium alginate microbeads. The obtained results reveal that the calcium alginate microbeads prepared through the ionotropic gelation technique possess great prominent for gallic acid encapsulation as well as its liberation.













Similar content being viewed by others
References
Soong YY, Barlow PJ (2006) Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem 97:524–530. https://doi.org/10.1016/j.foodchem.2005.05.033
Aydogdu A, Sumnu G, Sahin S (2019) Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohyd Polym 208:241–250. https://doi.org/10.1016/j.carbpol.2018.12.065
Omrani Z, Dadkhah TA (2020) New cyclodextrin-based supramolecular nanocapsule for codelivery of curcumin and gallic acid. Polym Bull 77:2003–2019. https://doi.org/10.1007/s00289-019-02845-5
Samooel J, Jun HC, Binna K, Hyejeong Y, Zbigniew AK, Cheorun J (2010) Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci 86:520–526. https://doi.org/10.1016/j.meatsci.2010.06.007
Chanwitheesuk A, Teerawutgulrag A, Kilburn JD, Rakariyatham N (2007) Antimicrobial gallic acid from caesalpinia mimosoides lamk. Food Chem 100:1044–1048. https://doi.org/10.1016/j.foodchem.2005.11.008
Punithavathi VR, Prince PSM, Kumar R, Selvakumari J (2011) Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol 650:465–471. https://doi.org/10.1016/j.ejphar.2010.08.059
Arunkumar S, Ilango K, Manikandan RS, Ramalakshmi N (2009) Synthesis and anti-inflammatory activity of some novel pyrazole derivatives of gallic acid. E-J Chem 6:S123–S128. https://doi.org/10.1155/2009/128586
Aranya M, Pensak J, Toshihiro A, Worapaka M, Jiradej M (2010) In vitro anti-aging activities of Terminalia chebula gall extract. Pharmaceutical Biology 48:469–481. https://doi.org/10.3109/13880200903586286
Chinmay P, Samik B, Sumanta D, Athar A, Manish G, Iqbal MS, Pallab M, Susanta SA, Bandyopadhyay U (2010) Gallic acid prevents nonsteroidal anti-inflammatory drug-induced gastropathy in rat by blocking oxidative stress and apoptosis. Free Radical Biol Med 49:258–267. https://doi.org/10.1016/j.freeradbiomed.2010.04.013
Hsieh-Hsun H, Chi-Sen C, Wei-Chi H, Sheng-You L, Cheng-Hsun W, Chau-Jong W (2010) Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-κB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem Toxicol 48:2508–2516. https://doi.org/10.1016/j.fct.2010.06.024
Fang Z, Bhandari B (2010) Encapsulation of polyphenols—a review. Trends Food Sci Technol 21:510–523. https://doi.org/10.1016/j.tifs.2010.08.003
Li J, Kim SY, Chen X, Park HJ (2016) Calcium-alginate beads loaded with gallic acid: preparation and characterization. LWT Food Sci Technol 68:667–673. https://doi.org/10.1016/j.lwt.2016.01.012
Yun PN, Simon S, Sudip R, Marija GN, Jianyong J, Conrad OP (2013) Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem 141:3192–3200. https://doi.org/10.1016/j.foodchem.2013.06.018
Yun PN, Sudip R, Jianyong J, Marija GN, Michel KN, Dongyan L, Siew YQ (2013) Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: a physicochemical study based on zein-gallic acid system. Food Chem 136:1013–1021. https://doi.org/10.1016/j.foodchem.2012.09.010
Dorniani D, Hussein MZB, Aminu UK, Fakurazi S, Abdul HS, Zalinah A (2012) Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int J Nanomed 7:5745–5756. https://doi.org/10.2147/IJN.S35746
Hu H, Nie L, Feng S, Suo J (2013) Preparation, characterization and in vitro release study of gallic acid loaded silica nanoparticles for controlled release. Pharmazie 68:401–405. https://doi.org/10.1691/ph.2013.2205
Paz R, Paula G, Reyes N, Chávez J, Santos J (2012) Acetylated starch and inulin as encapsulating agents of gallic acid and their release behaviour in a hydrophilic system. Food Chem 134:1–8. https://doi.org/10.1016/j.foodchem.2012.02.019
Cleonice GdR, Borges CD, Zambiazi RC, Nunes MR, Benvenutti EV, da Luz SR, D’Avila RF, Rutz JK (2013) Microencapsulation of gallic acid in chitosan, β-cyclodextrin and xanthan. Ind Crops Prod 46:138–146. https://doi.org/10.1016/j.indcrop.2012.12.053
Medina-Torres L, GarcÍa-Cruz EE, Calderas F, González Laredo RF, Sánchez-Olivares G, Gallegos-Infante JA, Rocha-Guzmán NE, RodrÍguez-RamÍrez J (2013) Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica). LWT Food Sci Technol 50:642–650. https://doi.org/10.1016/j.lwt.2012.07.038
Nagpal K, Singh SK, Mishra DN (2013) Nanoparticle mediated brain targeted delivery of gallic acid: in vivo behavioral and biochemical studies for protection against scopolamine-induced amnesia. Drug Delivery 20:112–119. https://doi.org/10.3109/10717544.2013.779330
Singh N, Chawla D, Singh J (2004) Influence of acetic anhydride on physicochemical, morphological and thermal properties of corn and potato starch. Food Chem 86:601–608. https://doi.org/10.1016/j.foodchem.2003.10.008
Stevens CV, Meriggi A, Booten K (2001) Chemical modification of inulin, a valuable renewable resource, and its industrial applications. Biomacromol 2:1–16. https://doi.org/10.1021/bm005642t
Peretz S, Anghel DF, Vasilescu E, Florea-Spiroiu M, Stoian C, Zgherea G (2015) Synthesis, characterization and adsorption properties of alginate porous beads. Polym Bull 72:3169–3182. https://doi.org/10.1007/s00289-015-1459-4
Pal P, Edathil AA, Banat F (2019) Calcium alginate gel and hard beads for the removal of total organic acid anions and heavy metal ions from industrial lean methyldiethanolamine solvent. Polym Bull 76:103–118. https://doi.org/10.1007/s00289-018-2376-0
Merakchi A, Bettayeb S, Drouiche N, Adour L, Lounici H (2019) Cross-linking and modification of sodium alginate biopolymer for dye removal in aqueous solution. Polym Bull 76:3535–3554. https://doi.org/10.1007/s00289-018-2557-x
Li M, Elder T, Buschle-Diller G (2017) Alginate-based polysaccharide beads for cationic contaminant sorption from water. Polym Bull 74:1267–1281. https://doi.org/10.1007/s00289-016-1776-2
Hu Y, Chen T, Dong X, Mei Z (2015) Preparation and characterization of composite hydrogel beads based on sodium alginate. Polym Bull 72:2857–2869. https://doi.org/10.1007/s00289-015-1440-2
Guo T, Zhang N, Huang J, Pei Y, Wang F, Tang K (2019) A facile fabrication of core–shell sodium alginate/gelatin beads for drug delivery systems. Polym Bull 76:87–102. https://doi.org/10.1007/s00289-018-2377-z
Loh QL, Wong YY, Choong C (2012) Combinatorial effect of different alginate compositions, polycations, and gelling ions on microcapsule properties. Colloid Polym Sci 290:619–629. https://doi.org/10.1007/s00396-011-2568-8
Aarstad O, Strand BL, Klepp-Andersen LM, Skjaìšk-Bræk G (2013) Analysis of G-block distributions and their impact on gel properties of in vitro epimerized mannuronan. Biomacromol 14:3409–3416. https://doi.org/10.1021/bm400658k
Nguyen LQT, Okajima M, Mitsumata T, Kan K, Tran HT, Kaneko T (2012) Trivalent metal-mediated gelation of novel supergiant sulfated polysaccharides extracted from Aphanothece stagnina. Colloid Polym Sci 290:163–172. https://doi.org/10.1007/s00396-011-2528-3
Berraaouan D, Elmiz M, Salhi S, Tahani A (2017) Effect of calcium chloride on rheological behavior of sodium alginate. Adv Mater Proc 2:629–633. https://doi.org/10.5185/amp.2017/893
Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P (1993) Development of a new drug carrier made from alginate. J Pharm Sci 82:912–917. https://doi.org/10.1002/jps.2600820909
Samaha D, Shehayeb R, Kyriacos S (2009) Modeling and comparison of dissolution profiles of diltiazem modified release formulations. Dissol Technol 16:41–46. https://doi.org/10.14227/DT160209P41
Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y (2003) Microsphere design for the colonic delivery of 5-fluorouracil. J Control Release 90:313–322. https://doi.org/10.1016/S0168-3659(03)00195-0
de Lima Souza J, Chiaregato CG, Faez R (2018) Green Composite based on PHB and montmorillonite for KNO3 and NPK delivery system. J Polym Environ 26:670–679. https://doi.org/10.1007/s10924-017-0979-4
Dash S, Murthy PN, Nath L, Chowdhury P (2010) Kinetic modeling on drug release from controlled drug delivery systems. Acta Poloniae Pharmaceut Drug Res 67:217–223
Sadoun O, Rezgui F, G’Sell C (2018) Optimization of valsartan encapsulation in biodegradables polyesters using Box-Behnken design. Mater Sci Eng, C 90:189–197. https://doi.org/10.1016/j.msec.2018.04.041
Behera AL, Patil SV, Sahoo SK (2011) Formulation and characteristics of 5flurouracil microspheres by solvent evaporation method. Int Pharm Pharmaceut Sc 3:32–35
Patel YL, Sher P, Pawar AP (2006) The effect of drug concentration and curing time on processing and properties of calcium alginate beads containing metronidazole by response surface methodology. AAPS PharmSciTech. https://doi.org/10.1208/pt070486
Maiti S, Dey P, Kaity S, Ray SMS, Biswanath S (2009) Investigation on processing variables for the preparation of fluconazole-loaded ethyl cellulose microspheres by modified multiple emulsion technique. AAPS PharmSciTech 10:703–715. https://doi.org/10.1208/s12249-009-9257-7
Venkatesan P, Manavalan R, Valliappan K (2011) Preparation and evaluation of sustained release loxoprofen loaded microspheres. J Basic Clin Pharm 2:159–15962
Marquette S, Peerboom C, Yates A, Denis L, Goole J, Amighi K (2014) Encapsulation of immunoglobulin G by solid-in-oil-in-water: effect of process parameters on microsphere properties. Eur J Pharm Biopharm 86:393–403. https://doi.org/10.1016/j.ejpb.2013.10.013
Lotfipour F, Mirzaeei S, Maghsoodi M (2012) Evaluation of the effect of CaCl2 and alginate concentrations and hardening time on the characteristics of Lactobacillus acidophilus loaded alginate beads using response surface analysis. Adv Pharmaceut Bull 2:71–78. https://doi.org/10.5681/apb.2012.010
Blandino A, Macías M, Cantero D (2001) Immobilization of glucose oxidase within calcium alginate gel capsules. Process Biochem 36:601–606. https://doi.org/10.1016/S0032-9592(00)00240-5
Blandino A, Maćas M, Cantero D (2000) Glucose oxidase release from calcium alginate gel capsules. Enzyme Microbial Technol 27:319–324. https://doi.org/10.1016/S0141-0229(00)00204-0
Uyen NTT, Hamid ZAA, Tram NXT, Ahmad N (2020) Fabrication of alginate microspheres for drug delivery: a review. Int J Biol Macromol 153:1035–1046. https://doi.org/10.1016/j.ijbiomac.2019.10.233
Nagarwal RC, Srinatha A, Pandit JK (2009) In situ forming formulation: development, evaluation, and optimization using 33 factorial design. AAPS PharmSciTech 10:977–984. https://doi.org/10.1208/s12249-009-9285-3
Deshmukh RK, Naik JB (2015) The impact of preparation parameters on sustained release aceclofenac microspheres: a design of experiments. Adv Powder Technol 26:244–252. https://doi.org/10.1016/j.apt.2014.10.004
Terzioǧlu P, Yücel S, Öztürk M (2017) Application of Box-Behnken design for modeling of lead adsorption onto unmodified and NaCl-modified zeolite NaA obtained from biosilica. Water Sci Technol 75:358–365. https://doi.org/10.2166/wst.2016.526
Gupta B, Poudel BK, Pathak S, Tak JW, Lee HH, Jeong JH, Choi HG, Yong CS, Kim JO (2016) Effects of formulation variables on the particle size and drug encapsulation of imatinib-loaded solid lipid nanoparticles. AAPS PharmSciTech 17:652–662. https://doi.org/10.1208/s12249-015-0384-z
Dahmoune F, Spigno G, Moussi K, Remini H, Cherbal A, Madani K (2014) Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind Crops Prod 61:31–40. https://doi.org/10.1016/j.indcrop.2014.06.035
Yetilmezsoy K, Demirel S, Vanderbei RJ (2009) Response surface modeling of Pb(II) removal from aqueous solution by Pistacia vera L.: box-Behnken experimental design. J Hazard Mater 171:551–562. https://doi.org/10.1016/j.jhazmat.2009.06.035
Honary S, Ebrahimi P, Hadianamrei R (2014) Optimization of particle size and encapsulation efficiency of vancomycin nanoparticles by response surface methodology. Pharm Dev Technol 19:987–998. https://doi.org/10.3109/10837450.2013.846375
Ali A, Shahriar H, Ahmad RM, Esmaeil D, Houshang AH (2014) Optimization of heavy metal removal from aqueous solutions by maghemite (γ-Fe2O3) nanoparticles using response surface methodology. J Geochem Explor 147:151–158. https://doi.org/10.1016/j.gexplo.2014.10.005
Heller J, Himmelstein KJ (1985) Poly (ortho ester) biodegradable polymer systems. Methods Enzymol 112:422–436
Zhao J, Li S, Zhao Y, Peng Z (2019) Effects of cellulose nanocrystal polymorphs and initial state of hydrogels on swelling and drug release behavior of alginate-based hydrogels. Polym Bull. https://doi.org/10.1007/s00289-019-02972-z
Salisu A, Sanagi MM, Abu Naim A, Abd Karim KJ, Wan Ibrahim WA, Abdulganiyu U (2016) Alginate graft polyacrylonitrile beads for the removal of lead from aqueous solutions. Polym Bull 73:519–537. https://doi.org/10.1007/s00289-015-1504-3
Li J, Lee IW, Shin GH, Chen X, Park HJ (2015) Curcumin-Eudragit® E PO solid dispersion: a simple and potent method to solve the problems of curcumin. Eur J Pharm Biopharm 94:322–332. https://doi.org/10.1016/j.ejpb.2015.06.002
López Córdoba A, Deladino L, Martino M (2013) Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohyd Polym 95:315–323. https://doi.org/10.1016/j.carbpol.2013.03.019
Soares JP, Santos JE, Chierice GO, Cavalheiro ETG (2004) Thermal behavior of alginic acid and its sodium salt. Eclet Quim 29:57–63. https://doi.org/10.1590/s0100-46702004000200009
Ross C, Rangika W, Luz S, MaryAnn A (2006) Synbiotic microcapsules that enhance microbial viability during nonrefrigerated storage and gastrointestinal transit. Appl Environ Microbiol 72:2280–2282. https://doi.org/10.1128/AEM.72.3.2280-2282
Lupo B, Maestro A, Gutiérrez JM, González C (2015) Characterization of alginate beads with encapsulated cocoa extract toprepare functional food: Comparison of two gelation mechanisms. Food Hydrocolloids 49:25–34. https://doi.org/10.1016/j.foodhyd.2015.02.023
Paris MJ, Ramírez-Corona N, Palou E, López-Malo A (2020) Modelling release mechanisms of cinnamon (Cinnamomum zeylanicum) essential oil encapsulated in alginate beads during vapor-phase application. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2020.110024
Jurić S, Đermić E, Topolovec-Pintarić S, Bedek M, Vinceković M (2019) Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J Integr Agri 18:2534–2548. https://doi.org/10.1016/S2095-3119(19)62634-1
Acknowledgements
The authors are sincerely thankful to MESRSFC, CNRST-Morocco and UMP for financial support of Project PPR 15-17 and PARA1-2019. The authors are thankful to the Professor Abdelmonaem Talhaoui, Head of Department of Chemistry, University of Mohammed first Oujda, for managing department of analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Essifi, K., Lakrat, M., Berraaouan, D. et al. Optimization of gallic acid encapsulation in calcium alginate microbeads using Box-Behnken Experimental Design. Polym. Bull. 78, 5789–5814 (2021). https://doi.org/10.1007/s00289-020-03397-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03397-9