Abstract
One-step synthesis of poly(MMA-b-CL) triarm block copolymers was carried out by atom transfer radical polymerization of methyl methacrylate (MMA) and ring-opening polymerization of ε-caprolactone (CL) using 3-chloro-1,2-propanediol trifunctional initiator. The triarm block copolymers comprising one poly-MMA arm and two poly-CL arms were synthesized by changing some polymerization conditions such as monomer/initiator concentration, polymerization time. The effect of the reactions conditions on the polydispersity and molecular weights was also investigated. The block lengths of the block copolymers were calculated by using 1H-nuclear magnetic resonance (1H-NMR) spectrum. It was observed that the block length could be altered by varying the monomer and initiator concentrations. The characterization of the products was achieved by using 1H-NMR, Fourier-transform infrared spectroscopy, gel-permeation chromatography, differential scanning calorimetry, thermogravimetric analysis and fractional precipitation techniques.
Similar content being viewed by others
Introduction
Macromolecules of a desired structure and molecular weight can be synthesized by controlled/“living” radical polymerization (CRP) techniques, such as atom transfer radical polymerization (ATRP) [1–5], nitroxide-mediated polymerization [6, 7], and reversible addition fragmentation chain transfer (RAFT) polymerization [8–19]. ATRP has a great number of advantages as compared with other CRPs. It includes a lot of monomers, and offers a general and efficient way to synthesize various (co)polymers [20], and does not require difficult conditions and has tolerance for functional groups and impurities [21, 22].
Block copolymers that have excellent physical properties are one of the most important polymeric materials used in technological applications and theoretical research because of their exceptional properties based on micro-phase separation [23–31]. The viscosity of a star block copolymer is higher than that of linear copolymer having the same molecular weight. Hence, star block copolymer is mostly used as a resistant material. There are a great number of excellent articles published on this subject [32–43].
In recent years, the one-step process has been successfully used for the synthesis of block and graft copolymers using different techniques. The process has more advantages than other popular methods. Due to the applicability of at least two transformation steps simultaneously, side reactions which lead to homopolymer formation are minimized [44–58]. Farah et al. carried out the synthesis of poly(ε-caprolactone-b-styrene) block copolymers through the combination of ATRP and ROP in the presence of 2-bromoisobutyryl bromide and bipyridine using N-methylpyrrolidione as the solvent [59]. Furthermore, various copolymers containing styrene [59–61], n-butyl acrylate [60], methyl methacrylate [61], tert-butyl acrylate [62], benzyl acrylate [62], ε-caprolactone [59, 60], l-lactide [61, 62] monomers were synthesized by a combination of the ATRP and ROP methods.
The present work is an extension of our recent studies involving the one-step synthesis of copolymers through simultaneous RAFT polymerization and ROP processes [17, 18, 63]. In this study, we synthesized poly (MMA-b-CL) triarm block copolymers using 3-cholor-1,2-propiondiol (ATRP-ROP initiator) by the simultaneous ATRP and ROP of the reactants in one-step. Star block copolymers synthesized could be used to prepare with the desired segment ratio by changing the polymerization conditions. The effect of the reactions conditions on the parameters was also investigated.
Experimental
Materials
3-Chloro-1,2-propanediol and copper(I) bromide (CuBr) were received from Aldrich and used as received. Dibutyltindilaurate (DBTDL) and petroleum ether were supplied by Merck and used as received. Benzene, chloroform, and tetrahydrofuran (THF) were received from Sigma-Aldrich and used as received. N,N,N′,N′,N″-Pentamethyldiethylenetriamine (PMDETA) was supplied by Fluka and used as received. Methanol and ethanol were received from Birpa and used as received. ε-Caprolactone (CL) was supplied by Alfa Aesar and used as received. MMA was received from Merck, which was purified as follows: it was washed with a 10 wt% aqueous NaOH solution, dried over anhydrous CaCl2 overnight, and distilled over CaH2 under reduced pressure before use. All other chemicals were reagent grade and used as received.
Instrumentation
The molecular weights and molecular weight distributions were measured with Malvern Viscotek RI-UV-GPC max gel-permeation chromatography (GPC) with THF as the solvent. A calibration curve was generated with four polystyrene standards: 2960, 50,400, and 696,500 Da, of low polydispersity. Fourier-transform infrared (FTIR) spectra were recorded using an Alpha-p Bruker model FTIR spectrometer. 1H-nuclear magnetic resonance (1H-NMR) spectra of the samples in CDCl3 as the solvent, with tetra methylsilane as the internal standard, were recorded using a Bruker Ultra Shield Plus, ultra-long hold time 400 MHz NMR spectrometer. Thermogravimetric analysis (TGA) measurements of the polymers were carried out under nitrogen using a Perkin Elmer Pyris 1 TGA and Spectrum thermal analyzer to determine thermal degradation. Differential scanning calorimetry (DSC) measurements were carried out by using a Perkin Elmer Diamond DSC series thermal analysis system. Dried sample was heated at a rate of 10 °C/min under nitrogen atmosphere.
One-step polymerization
Poly(MMA-b-CL) triarm block copolymers were synthesized using two different monomers in one-step process. Specified amounts of ATRP-ROP initiator, MMA, CL, DBTDL (catalyst for ROP of CL), PMDETA, CuBr, and benzene (as solvent) were charged separately into a Pyrex tube, and subsequently argon was purged into the tube through a needle. The tube was tightly capped with a rubber septum and was dropped into an oil bath thermostated at 110 °C for fixed time. After the polymerization, the reaction mixture was poured into an excess of methanol to separate the block copolymers. The copolymers were dried at 40 °C under vacuum for 3 days. The yield of the polymer was determined gravimetrically.
Fractional precipitations of the polymers
Fractional precipitations (γ) of the polymers were carried out according to the procedure reported in the literature [63–65]. Vacuum-dried polymer sample (approximately 0.5 g) was dissolved in 5 mL of THF. Petroleum ether was added as drop wise to the solution with stirring until turbidity occurs. At this point, 1–2 mL of petroleum ether was added to complete the precipitation. The precipitate was removed by filtration. The solvent was THF and the nonsolvent was petroleum ether. In this solvent–nonsolvent system, the γ values were calculated as the ratios of the total volume of nonsolvent used for the first fraction to the volume of solvent used.
The nonsolvent addition into the filtrate solution was continued according to the same procedure mentioned above to determine the γ value for the second fraction if there is.
Results and discussion
One-step polymerization for poly(MMA-b-CL) triarm block copolymers
The one-step polymerization of a vinyl monomer and a lactone initiated by ATRP-ROP initiator is shown in Scheme 1. This process creates two new active sites—a site on an equal number of hydroxyl group for ROP reaction and a chloride group for ATRP. During this one-pot synthesis, ATRP of MMA is carried out simultaneously as ROP of CL proceeds, to yield the block copolymer. The effects of polymerization time, initiator concentration, and monomer concentration on the copolymerization in the presence of ATRP-ROP initiator by the application of simultaneous ATRP and ROP processes have been studied. The results of the one-step polymerization of MMA and CL are shown in Tables 1, 2, 3. The monomer conversion was calculated from the weight of recovered polymer. The conversion of monomer was between 13.42 and 47.73 wt%. Increases in the molecular weights of the copolymers as compared with that of the initiator can confirm block copolymer formation.
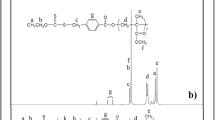
The FTIR spectrum of 3-chloro-1,2-propanediol in Fig. 1a shows 3321 cm−1 for –OH groups, 2884–2953 cm−1 for aliphatic –CH2 and –CH groups, 1031 cm−1 for –C–O groups, 704 cm−1 for –Cl groups. The FTIR spectrum of the triblock copolymer is shown in Fig. 1b. The signals at 2949–2993 cm−1 for aliphatic –CH2 and –CH3, 1722 cm−1 for –C=O, 1140 cm−1 for –C–O of the copolymer appear in the FTIR spectra. The –OH signal diminishes at the FTIR spectrum of the copolymer (Fig. 1b) according to the –OH signal of the initiator (Fig. 1a). The 1H-NMR spectrum of 3-chloro-1,2-propanediol in Fig. 2a shows the 3.6 ppm for –OH protons, 3.9 and 4.0 ppm for –CH2 and –CH protons, 4.0 and 5.0 ppm for –OCH2 protons. Typical 1H-NMR spectra of the copolymer in Fig. 2b show 0.7 ppm for –CH3 protons of poly-MMA segment, 0.9 ppm for –CH2 protons of poly-MMA segment, 1.1 ppm for –OH protons of poly-CL segment, 1.3 ppm for –CH and –CH2 protons of 3-chloro-1,2-propanediol, 1.8 ppm for –OCH2 protons of poly-CL segment, 3.5 ppm for –OCH3 protons of poly-MMA segment.
The effect of the polymerization time on the one-step block copolymerization is presented in Table 1. Polymerization time dependence of M n on the one-step copolymerization is shown in Fig. 3. First, longer polymerization times cause higher polymer molecular weights. Second, the polymers with lower molecular weights are obtained for polymerizations of longer durations. Longer polymerization times cause higher polymer yields. Higher amounts of ATRP-ROP initiator cause a higher polymer yield (Table 2). Interestingly, the value of M n can only decrease if new chains are generated. However, that is not in accordance with a controlled polymerization. Increased amounts of initiator in the reaction mixture lead to the formation of a higher number of active centers. Consequently, increased numbers of growing radicals are formed in the system. Hence, it may be expected that they have shorter poly-MMA and poly-CL segments, which is confirmed by a decrease in the molecular weights of the block copolymers, as shown in Table 2. The same situation was also observed in our previous articles [16, 17, 63]. Dependence of ATRP-ROP initiator concentration on M n for the one-step copolymerization is shown in Fig. 4. Increasing the amount of monomers also causes an increase in both the yield and the molecular weights of the copolymers as expected (Table 3). Dependence of MMA concentration on M n for the copolymerization is shown in Fig. 5. The M w/M n values of the triarm block copolymers are between 1.98 and 3.23 (Tables 1, 2, 3). Because more than one propagating center initiates the polymerization, the M w/M n values of the block copolymers are relatively higher than expected. Because DBTDL, ROP catalyst of CL, can interfere with the radical polymerization of MMA, the block copolymers with very broad molecular weight distributions can be formed. All GPC chromatograms were unimodal and indicated more the molecular weight values of block copolymers than that of ATRP-ROP initiator. For example, Fig. 6 shows the unimodal GPC curves of the block copolymers (MB-3, MB-4, MB-5, and MB-6 in Table 3). The polymer composition of the copolymers was calculated using the integral ratios of the signals corresponding to the –OCH3 groups of poly-MMA (δ = 3.5 ppm), –OCH2 groups of poly-CL (δ = 1.8 ppm). The poly-MMA content of copolymers was more than the poly-CL content. Generally, the values of polymer composition of the copolymers did not change as shown Tables 1, 2, 3.
Thermal analysis of poly(MMA-b-CL) triarm block copolymers
Thermal analysis of the samples was carried out by taking DSC, and TGA curves. All samples exhibited glass transition temperatures (T g). The reported T g values were obtained from the second heating curves. T g value of the block copolymer (BA-6) was 5 °C (Fig. 7). T g values were reported in the literature for homo poly-CL, and homo poly-MMA as −72 °C [66, 67], and 105 °C [68], respectively. The T g value observed by DSC appears between T g of the poly-MMA homopolymer and T g of the poly-CL homopolymer. The only one T g value for the sample shows the miscible nature of the related homopolymers. The same situation (the observation of only one glass transition) can also be seen in our previous articles [18, 63]. Similarly, TGA showed that in the block copolymers, poly-MMA, and poly-CL blocks did not have individual decomposition temperatures (T d) (Fig. 8). TGA showed interesting properties of the block copolymer indicating continuous weight loss starting from 13 °C to nearly 430 °C with a derivative at 375 °C. The first decomposition observed at about 200 °C may have been caused by the solvent traces. One main individual T d of the block copolymers can be attributed to the high miscibility of the polymerizable methacrylate groups of poly-MMA with poly-CL moieties of the copolymers.
Fractional precipitation
The fractional precipitation (γ) values of poly(MMA-b-CL) block copolymers were between 0.42 and 0.68. In the solvent–nonsolvent system, γ values were found to be 0.50–0.55 for homo poly-MMA [16], 1.02–1.20 for homo poly-CL [18]. The γ values of the block copolymers were generally between that of homo poly-MMA and that of homo poly-CL. Fractional precipitation behavior can give an evidence for the formation of block copolymer.
Conclusions
One-step synthesis of block copolymer was carried out ATRP of MMA and ROP of CL using 3-cholor-1,2-propiondiol initiator. The initiator has demonstrated the characteristic initiator behavior in the copolymerization of MMA and CL. A set of one-step synthesis, and ATRP and ROP conditions of triarm block copolymers, poly(MMA-b-CL), were evaluated. The block copolymers were relatively obtained in high yield and molar weight. The proposed procedure for the preparation of block copolymers is simple and efficient. Basically, controlling the polymerization parameters such as ATRP-ROP initiator concentration, monomer concentration, and polymerization time, ATRP-ROP initiator can be promising materials in order to obtain block copolymers.
References
Wang JS, Matyjaszewski K (1995) Controlled living radical polymerization: atom-transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc 117:5614–5615. doi:10.1021/ja00125a035
Kato M, Kamigaito M, Sawomoto M, Higashimura T (1995) Polymerization of methyl-methacrylate with the carbon-tetrachloride dichloro tris(triphenylphosphine)ruthenium(II) methyl aluminum bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules 28:1721–1723. doi:10.1021/ma00109a056
Haddleton DM, Waterson C, Derrick PJ, Jasieczek CB, Shooter AJ (1997) Monohydroxy terminally functionalised poly(methyl methacrylate) from atom transfer radical polymerisation. Chem Commun 7:683–684. doi:10.1039/a700677b
Lee HI, Matyjaszewski K, Yu S, Sheiko SS (2005) Molecular brushes with spontaneous gradient by atom transfer radical polymerization. Macromolecules 38:8264–8271. doi:10.1021/ma051231z
Kruk M, Dufour B, Celer EB, Kowalewski T, Jaroniec M, Matyjaszewski K (2005) Synthesis of mesoporous carbons using ordered and disordered mesoporous silica templates and polyacrylonitrile as carbon precursor. Phys Chem B 109:9216–9225. doi:10.1021/jp045594x
Hawker CJ, Bosman AW, Harth E (2001) New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev 101:3661–3688. doi:10.1021/cr990119u
Lessard B, Maric M (2008) Nitroxide-mediated synthesis of poly(poly(ethylene glycol) acrylate) (PPEGA) comb-like homopolymers and block copolymers. Macromolecules 41:7870–7880. doi:10.1021/ma800603a
Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad E, Rizzardo E, Thang SH (1998) Living free-radical polymerization by reversible addition-fragmentation chain transfer: the RAFT process. Macromolecules 31:5559–5562. doi:10.1021/ma9804951
Ozturk T, Hazer B (2010) Synthesis and characterization of a novel macromonomer initiator for reversible addition fragmentation chain transfer (RAFT). Evaluation of the polymerization kinetics and gelation behaviors. J Macromol Sci Part A Pure Appl Chem 47:265–272. doi:10.1080/10601320903527095
Şanal T, Oruç O, Öztürk T, Hazer B (2015) Synthesis of pH- and thermo-responsive poly (ε-caprolactone-b-4-vinyl benzyl-g-dimethyl amino ethyl methacrylate) brush type graft copolymers via RAFT polymerization. J Polym Res 22:3–15. doi:10.1007/s10965-014-0640-z
Moad G, Chong YK, Postma A, Rizzardo E, Thang SH (2005) Advances in RAFT polymerization: the synthesis of polymers with defined end-groups. Polymer 46:8458–8468. doi:10.1016/j.polymer.2004.12.061
Perrier S, Davis TP, Carmichael AJ, Haddleton DM (2003) Reversible addition-fragmentation chain transfer polymerization of methacrylate, acrylate and styrene monomers in 1-alkyl-3-methylimidazolium hexafluoro phosphate. Eur Polym J 39:417–422. doi:10.1016/S0014-3057(02)00250-1
Barner Kowollik C, Quinn JF, Morsley DR, Davis TP (2001) Modeling the reversible addition-fragmentation chain transfer process in cumyldithiobenzoate-mediated styrene homo polymerizations: assessing rate coefficients for the addition-fragmentation equilibrium. J Polym Sci Part A: Polym Chem 39:1353–1365. doi:10.1002/pola.1112
Ray B, Isobe Y, Matsumoto K, Habaue S, Okamoto Y, Kamigaito M, Sawamoto M (2004) RAFT polymerization of N-isopropylacrylamide in the absence and presence of Y(OTf)(3): simultaneous control of molecular weight and tacticity. Macromolecules 37:1702–1710. doi:10.1021/ma035119h
Patton DL, Advincula RC (2006) A versatile synthetic route to macromonomers via RAFT polymerization. Macromolecules 39:8674–8683. doi:10.1021/ma061382h
Öztürk T, Göktaş M, Hazer B (2011) Synthesis and characterization of poly(methyl methacrylate-block-ethylene glycol-block-methyl methacrylate) block copolymers by reversible addition fragmentation chain transfer polymerization. J Macromol Sci Part A Pure Appl Chem 48:65–72. doi:10.1080/10601325.2011.528310
Öztürk T, Göktaş M, Hazer B (2010) One-step synthesis of triarm block copolymers via simultaneous reversible-addition fragmentation chain transfer and ring-opening polymerization. J Appl Polym Sci 117:1638–1645. doi:10.1002/app.32031
Öztürk T, Atalar MN, Göktaş M, Hazer B (2013) One-step synthesis of block-graft copolymers via simultaneous reversible-addition fragmentation chain transfer and ring-opening polymerization using a novel macroinitiator. J Polym Sci Part A: Polym Chem 51:2651–2659. doi:10.1002/pola.26654
Öztürk T, Göktaş M, Savaş B, Işıklar M, Atalar MN, Hazer B (2014) Synthesis and characterization of poly(vinylchloride-graft-2-vinylpyridine) graft copolymers using a novel macroinitiator by reversible addition-fragmentation chain transfer polymerization. e-Polymers 14:27–34. doi:10.1515/epoly-2013-0011
Wang JS, Matyjaszewski K (1995) Controlled living radical polymerization—halogen atom-transfer radical polymerization promoted by a Cu(I)Cu(Ii) redox process. Macromolecules 28:7901–7910. doi:10.1021/ma00127a042
Patten TE, Matyjaszewski K (1998) Atom transfer radical polymerization and the synthesis of polymeric materials. Adv Mater 10:901–915. doi:10.1002/(SICI)1521-4095(199808)10:12<901:AID-ADMA901>3.3.CO;2-2
Zhang H, Lei X, Su Z, Liu P (2007) A novel method of surface-initiate atom transfer radical polymerization of styrene from silica nanoparticles for preparation of mono dispersed core-shell hybrid nanospheres. J Polym Res 14:253–260. doi:10.1007/s10965-007-9104-z
Yagci Y, Schnabel W (1990) Light-induced synthesis of block and graft copolymers. Prog Polym Sci 15:551–601. doi:10.1016/0079-6700(90)90006-M
Ruzette AV, Leibler L (2005) Block copolymers in tomorrow’s plastics. Nat Mater 4:19–31. doi:10.1038/nmat1295
Shipp DA, Wang J, Matyjaszewski K (1998) Synthesis of acrylate and methacrylate block copolymers using atom transfer radical polymerization. Macromolecules 31:8005–8008. doi:10.1021/ma981033q
Bates FM, Fredrickson GH (1990) Block copolymer thermodynamics: theory and experiment. Ann Rev Phys Chem 41:525–557. doi:10.1146/annurev.pc.41.100190.002521
Bilalis P, Pitsikalis M, Hadjichristidis N (2006) Controlled nitroxide mediated and reversible addition-fragmentation chain transfer polymerization of N-vinylpyrrolidone: synthesis of block copolymers with styrene and 2-vinylpyridine. J Polym Sci Part A Polym Chem 44:659–665. doi:10.1002/pola.21198
Noshay A, Mcgrath JE (1977) Block copolymers, overview and critical survey. Academic Press, New York
Makarova LI, Filimonova LV, Dubrovina LV, Buzin MI, Nikiforova GG, Zavin BG, Papkov VS (2010) Synthesis and properties of siloxane(ethylene oxide) urethane block copolymers. Polym Sci Ser B 52:346–352. doi:10.1134/S1560090410050118
Vinchon Y, Reeb R, Riess G (1976) Preparation of macromolecular azo initiators by anionic-polymerization: application to synthesis of block copolymers. Eur Polym J 12:317–321
Peng ZP, Wang D, Liu X, Tong Z (2007) RAFT synthesis of a water-soluble triblock copolymer of poly(styrenesulfonate)-b-poly(ethylene glycol)-b-poly(styrenesulfonate) using a macromolecular chain transfer agent in aqueous solution. J Polym Sci Part A Polym Chem 45:3698–3706. doi:10.1002/pola.22119
Yoshikawa C, Goto A, Tsujii Y, Fukuda T, Yamamoto K, Kishida A (2005) Fabrication of high-density polymer brush on polymer substrate by surface-initiated living radical polymerization. Macromolecules 38:4604–4610. doi:10.1021/ma047556h
Baum M, Brittain WJ (2002) Synthesis of polymer brushes on silicate substrates via reversible addition fragmentation chain transfer technique. Macromolecules 35:610–615. doi:10.1021/ma0112467
Shinoda H, Matyjaszewski K (2001) Improving the structural control of graft copolymers. Copolymerization of poly (dimethyl siloxane) macromonomer with methyl methacrylate using RAFT polymerization. Macromol Rapid Commun 22:1176–1181. doi:10.1002/1521-3927(20011001)22:14<1176:AID-MARC1176>3.0.CO;2-J
Lord HT, Quinn JF, Angus SD, Whittaker MR, Stenzel MH, Davis TP (2003) Micro gel stars via reversible addition fragmentation chain transfer (RAFT) polymerisation: a facile route to macro porous membranes, honeycomb patterned thin films and inverse opal substrates. J Mater Chem 13:2819–2824. doi:10.1039/b304208c
Chen M, Ghiggino KP, Launikonis A, Mau AWH, Rizzardo E, Sasse WHF, Thang SH, Wilson GJ (2003) RAFT synthesis of linear and star-shaped light harvesting polymers using di- and hexafunctional ruthenium polypyridine reagents. J Mater Chem 13:2696–2700. doi:10.1039/b303576j
Roy D, Guthrie JT, Perrier S (2005) Graft polymerization: grafting poly(styrene) from cellulose via reversible addition-fragmentation chain transfer (RAFT) polymerization. Macromolecules 38:10363–13372. doi:10.1021/ma0515026
Bosman AW, Vestberg R, Heumann A, Frechet JMJ, Hawker CJJ (2003) A modular approach toward functionalized three-dimensional macromolecules: from synthetic concepts to practical applications. Am Chem Soc 125:715–728. doi:10.1021/ja028392s
Haraguchi N, Hirao A (2003) Synthesis of well-defined star-linear block polystyrenes by coupling reaction of chain-functionalized polystyrenes with a definite number of benzyl bromide moieties with polystyryllithiums. Macromolecules 36:9364–9372. doi:10.1021/ma034799l
Li Z, Hillmyer MA, Lodge TP (2004) Synthesis and characterization of triptych mu-ABC star triblock copolymers. Macromolecules 37:8933–8940. doi:10.1021/ma048607d
Fragouli PG, Iatrou H, Hadjichristidis N, Sakurai T, Hirao A (2006) Synthesis and characterization of model 3-miktoarm star copolymers of poly(dimethylsiloxane) and poly(2-vinylpyridine). J Polym Sci Polym Chem 44:614–619. doi:10.1002/pola.21197
Hazer B (1991) Synthesis of styrene-tetrahydrofuran branched block copolymers. Eur Polym J 27:975–978. doi:10.1016/0014-3057(91)90043-N
Le Hellaye M, Lefay C, Davis TP, Stenzel MH, Barner- Kowollik C (2008) Simultaneous reversible addition fragmentation chain transfer and ring-opening polymerization. J Polym Sci Part A Polym Chem 46:3058–3067. doi:10.1002/pola.22647
Hong J, Wang Q, Fan Z (2006) Synthesis of multiblock polymer containing narrow polydispersity blocks. Macromol Rapid Commun 27:57–62. doi:10.1002/marc.200500678
Cheng C, Khoshdel E, Wooley KL (2007) One-pot tandem synthesis of a core: shell brush copolymer from small molecule reactants by ring-opening metathesis and reversible addition-fragmentation chain transfer (co)polymerizations. Macromolecules 40:2289–2292. doi:10.1021/ma0627525
Mahanthappa MK, Bates FS, Hillmyer MA (2005) Synthesis of ABA triblock copolymers by a tandem ROMP-RAFT strategy. Macromolecules 38:7890–7894. doi:10.1021/ma051535l
Mori H, Masuda S, Endo T (2008) Ring-opening copolymerization of 10-methylene-9,10-dihydroanthryl-9-spirophenylcyclopropane via free radical and RAFT processes. Macromolecules 41:632–639. doi:10.1021/ma0714262
Han DH, Pan CY (2007) Preparation and characterization of heteroarm H-shaped terpolymers by combination of reversible addition-fragmentation transfer polymerization and ring-opening polymerization. J Polym Sci Part A Polym Chem 45:789–799. doi:10.1002/pola.21907
Xu XQ, Jia ZF, Sun RM, Huang JL (2006) Synthesis of well-defined, brush-type, amphiphilic [poly(styrene-co-2-hydroxyethyl methacrylate)-graft-poly(epsilon-caprolactone)]-b-poly(ethylene oxide)-b-[poly(styrene-co-2-hydroxyethyl methacrylate)-graft-poly(epsilon-caprolactone)] and its aggregation behavior in aqueous media. J Polym Sci Part A: Polym Chem 44:4396–4408. doi:10.1002/pola.21549
Xu XW, Huang JL (2006) Synthesis and characterization of amphiphilic copolymer of linear poly(ethylene oxide) linked with [poly(styrene-co-2-hydroxyethyl methacrylate)graft-poly(epsilon-caprolactone)] using sequential controlled polymerization. J Polym Sci Part A Polym Chem 44:467–476. doi:10.1002/pola.21162
Liu J, Pan CY (2005) Synthesis and characterization of H-shaped copolymers by combination of RAFT polymerization and CROP. Polymer 46:11133–11141. doi:10.1016/j.polymer.2005.08.072
Wang WP, You YZ, Hong CY, Xu J, Pan CY (2005) Synthesis of comb-shaped copolymers by combination of reversible addition-fragmentation chain transfer polymerization and cationic ring-opening polymerization. Polymer 46:9489–9494. doi:10.1016/j.polymer.2005.07.037
Shi PJ, Li YG, Pan CY (2004) Block and star block copolymers by mechanism transformation: X. Synthesis of poly(ethylene oxide) methyl ether/polystyrene/poly(L-lactide) ABC miktoarm star copolymers of by combination of RAFT and ROP. Eur Polym J 40:1283–1290. doi:10.1016/j.europolymj.2004.02.024
You Y, Hong C, Wang W, Lu W, Pan CY (2004) Preparation and characterization of thermally responsive and biodegradable block copolymer comprised of PNIPAAM and PLA by combination of ROP and RAFT methods. Macromolecules 37:9761–9767. doi:10.1021/ma048444t
Chang C, Wei H, Quan CY, Li YY, Liu J, Wang ZC, Cheng SX, Zhang XZ, Zhuo RX (2008) Fabrication of thermosensitive PCL-PNIPAAm-PCL triblock copolymeric micelles for drug delivery. J Polym Sci Part A: Polym Chem 46:3048–3057. doi:10.1002/pola.22645
Luan B, Zhang BQ, Pan CY (2006) Synthesis and characterizations of well-defined branched polymers with AB(2) branches by combination of RAFT polymerization and ROP as well as ATRP. J Polym Sci Part A: Polym Chem 44:549–560. doi:10.1002/pola.21183
Schmid C, Falkenhagen J, Barner-Kowollik C (2011) An efficient avenue to poly(styrene)-block-poly(epsilon-caprolactone) polymers via switching from RAFT to hydroxyl functionality: synthesis and characterization. J Polym Sci Part A: Polym Chem 49:1–10. doi:10.1002/pola.24299
Yu YC, Li G, Kang HU, Youk JH (2012) One-step synthesis of poly(alkyl methacrylate)-b-polyester block copolymers via a dual initiator route combining RAFT polymerization and ROP. Colloid Polym Sci 290:1707–1712. doi:10.1007/s00396-012-2788-6
Farah AA, Hall N, Morin S, Pietro W-J (2006) Poly(epsilon-caprolactone)-block-polystyrene metallopolymers via sequential ROP and ATRP condition with in situ generated ruthenium catalyst. Polymer 47:4282–4291. doi:10.1016/j.polymer.2006.03.037
Deng G, Zhang L, Liu C, He L, Chen Y (2005) Synthesis of miktoarm star (block) polymers based on a heterofunctional initiator via combination of ROP, ATRP and functional group transformation. Eur Polym J 41:1177–1186. doi:10.1016/j.eurpolymj.2005.01.003
Tao L, Luan B, Pan C-Y (2003) Block and star block copolymers by mechanism transformation. VIII Synthesis and characterization of triblock poly(LLA-b-St-b-MMA) by combination of ATRP and ROP. Polymer 44:1013–1020. doi:10.1016/S0032-3861(02)00870-4
Messman J-M, Scheuer A-D, Storey R-F (2005) Synthesis and characterization of A–B–A triblock copolymers derived from chloro-telechelic poly(L-lactide): combining ring-opening polymerization (ROP) and atom transfer radical polymerization (ATRP). Polymer 46:3628–3638. doi:10.1016/j.polymer.2005.03.023
Göktaş M, Öztürk T, Atalar MN, Tekeş AT, Hazer B (2014) One-step synthesis of triblock copolymers via simultaneous reversible-addition fragmentation chain transfer (RAFT) and ring-opening polymerization using a novel difunctional macro-RAFT agent based on polyethylene glycol. J Macromol Sci Part A 51:854–863. doi:10.1080/10601325.2014.953366
Hazer B, Erdem B, Lenz RW (1994) Styrene polymerization with some new macro or macromonomeric azo initiators having PEG units. J Polym Sci Part A: Polym Chem 32:1739–1746. doi:10.1002/pola.1994.080320916
Wu B, Lenz RW, Hazer B (1999) Polymerization of methyl methacrylate and its copolymerization with epsilon-caprolactone catalyzed by isobutylalumoxane catalyst. Macromolecules 32:6856–6859. doi:10.1021/ma990166o
Shuster M, Narkis M, Siegmann A (1994) Polymeric antiplasticization of polycarbonate with polycaprolactone. Polym Eng Sci 34:1613–1618. doi:10.1002/pen.760342106
Huarng JC, Min KS, White JL (1988) Phase-equilibrium in the binary and ternary blend system—polycaprolactone-polyvinyl chloride-styrene acrylonitrile copolymer. Polym Eng Sci 28:1590–1599. doi:10.1002/pen.760282404
Brandrup J, Immergut EH, Grulke EA (2003) Polymer handbook, 4th edn. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öztürk, T., Yavuz, M., Göktaş, M. et al. One-step synthesis of triarm block copolymers by simultaneous atom transfer radical and ring-opening polymerization. Polym. Bull. 73, 1497–1513 (2016). https://doi.org/10.1007/s00289-015-1558-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1558-2