Abstract
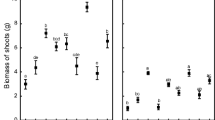
In the present study, the impact of co-inoculation of arbuscular mycorrhizal fungi (AM Rhizophagus sp., NCBI-MN710507) and Zinc solubilizing bacteria (ZSB2- Bacillus megaterium, NCBI-KY687496) on plant growth, soil dehydrogenase activity, soil respiration and the changes in bacterial diversity in rhizosphere of turmeric (Curcuma longa) were examined. Our results showed that higher plant height and dry biomass were observed in treatments co-inoculated with AM and ZSB2. Likewise, dehydrogenase activity and soil respiration were more significant in the co-inoculation treatment, indicating abundance of introduced as well as inherent microflora. Bacterial community analysis using 16S rRNA revealed changes in the structure and diversity of various taxa due to co-inoculation of AM and ZSB2. Alpha diversity indexes (Shannon and Chao1) and beta diversity indexes obtained through unweighted unifrac approach also showed variation among the treated samples. Chloroflexi was the dominant phylum followed by Proteobacteria, Actinobacteria and Acidobacteria which accounted for 80% of all treated samples. The composition of bacterial communities at genus level revealed that co-inoculation caused distinct bacterial profiles. The Linear discriminant analysis effect size revealed the dominance of ecologically significant genera such as Bradyrhizobium, Candidatus, Pedomicrbium, Thermoporothrix, Acinetobacter and Nitrospira in treatments co-inoculated with AM and ZSB2. On the whole, co-inoculated treatments revealed enhanced microbial activities and caused significant positive shifts in the bacterial diversity and abundance compared to treatments with sole application of ZSB2 or AM.





Similar content being viewed by others
References
Aarthi S, Suresh J, Leela NK, Prasath D (2020) Multi environment testing reveals genotype-environment interaction for curcuminoids in turmeric (Curcuma longa L.). Ind Crops Prod 145:112090. https://doi.org/10.1016/j.indcrop.2020.112090
Trabelsi D, Mhamdi R (2013) Microbial inoculants and their impact on soil microbial communities: a review. Biomed Res Int. https://doi.org/10.1155/2013/863240
Raklami A, Bechtaoui N, Tahiri A, Anli M, Meddich A, Oufdou K (2019) Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front Microbiol 10:1106. https://doi.org/10.3389/fmicb.2019.01106
Wang YY, Yin QS, Qu Y, Li GZ, Hao L (2018) Arbuscular mycorrhiza-mediated resistance in tomato against Cladosporium fulvum induced mould disease. J Phytopathol 166:67–74. https://doi.org/10.1111/jph.12662
Rivero J, Álvarez D, Flors V, Azcón-Aguilar C, Pozo MJ (2018) Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol 220:1322–1336. https://doi.org/10.1111/nph.15295
Shi Z, Wang F, Liu Y (2012) Response of soil respiration under different mycorrhizal strategies to precipitation and temperature. J Soil Sci Plant Nutr 12(3):411–420. https://doi.org/10.4067/S0718-95162012005000003
Rouphael Y, Franken P, Schneider C, Schwarz D, Giovannetti M, Agnolucci M, De Pascale S, Bonini P, Colla G (2015) Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci Hortic 196:91–108. https://doi.org/10.1016/j.scienta.2015.09.002
Sarathambal C, Ilamurugu K, Balachandar D, Chinnadurai C, Gharde Y (2015) Characterization and crop production efficiency of diazotrophic isolates from the rhizosphere of semi-arid tropical grasses of India. Appl Soil Ecol 87:1–10. https://doi.org/10.1016/j.apsoil.2014.11.004
Rashid MI, Mujawar LH, Shahzad T, Almeelbi T, Iqbal MI, Mohammad O (2016) Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res 183:26–41. https://doi.org/10.1016/j.micres.2015.11.007
Turrini A, Avio L, Giovannetti M, Agnolucci M (2018) Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: the challenge of translational research. Front Plant Sci 9:1407. https://doi.org/10.3389/fpls.2018.01407
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778. https://doi.org/10.1093/jxb/eri197
OrdoñeznYM FBR, Lara LR, Rodriguez A, Uribe- Vélez D, Sanders IR (2016) Bacteria with phosphate solubilizing capacity alter mycorrhizal fungal growth both inside and outside the root and in the presence of native microbial communities. PLoS ONE 11:e0154438. https://doi.org/10.1371/journal.pone.0154438
Song X, Liu M, Wu D, Griffiths B, Jiao J, Li H, Hu F (2015) Interaction matters: synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field. Appl Soil Ecol 89:25–34. https://doi.org/10.1016/j.apsoil.2015.01.005
Sarathambal C, Khankhane PJ, Gharde Y, Kumar B, Varun M, Arun S (2017) The effect of Plant growth promoting rhizobacteria on the growth, physiology, and Cd uptake of Arundo donax L. Int J Phytoremediation 19(4):360–370. https://doi.org/10.1080/15226514.2016.1225289
Dinesh R, Srinivasan V, Hamza S, Sarathambal C, Anke Gowda SJ, Ganeshamurthy AN, Gupta SB, Aparna Nair V, Subila KP, Lijina A, Divya VC (2018) Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321:173–186. https://doi.org/10.1016/j.geoderma.2018.02.013
Liu Y, Sun Q, Li J, Lian B (2018) Bacterial diversity among the fruit bodies of ectomycorrhizal and saprophytic fungi and their corresponding hyphosphere soils. Sci Rep 8:11672. https://doi.org/10.1038/s41598-018-30120-6
Casida LE Jr, Klein DA, Santoro R (1964) Soil dehydrogenase activity. Soil Sci 98:371–378. https://doi.org/10.1097/00010694-196412000-00004
Phillips JM, Hayman DS (1970) Improved produces for clearing roots and staining parasitic and VAM fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/S0007-1536(70)80110-3
Bukin Y, Galachyants Y, Morozov I (2019) The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci Data 6:190007. https://doi.org/10.1038/sdata.2019.7
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335. https://doi.org/10.1038/nmeth.f.303
Rognes T, Flouri T, Nichols B, Quince C, Mahe F (2016) V search a versatile open source tool for metagenomics. Peer J. https://doi.org/10.7717/peerj.2584
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Dinesh R, Anandaraj A, Kumar A, Bini YK, Subila KP, Aravind R (2015) Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol Res 173:34–43. https://doi.org/10.1016/j.micres.2015.01.014
Bourles A, Guentas L, Charvis C (2020) Co-inoculation with a bacterium and arbuscular mycorrhizal fungi improves root colonization, plant mineral nutrition, and plant growth of a Cyperaceae plant in an ultramafic soil. Mycorrhiza 30:121–131. https://doi.org/10.1007/s00572-019-00929-8
Medina A, Probanza A, Gutierrez Mañero FJ, Azcón R (2003) Interactions of arbuscular-mycorrhizal fungi and Bacillus strains and their effects on plant growth, microbial rhizosphere activity (thymidine and leucine incorporation) and fungal biomass (ergosterol and chitin). Appl Soil Ecol 22:15–28. https://doi.org/10.1186/s40694-019-0086-5
Adriana MA, Rosario A, Juan RL, Ricardo A (2007) Differential effects of a Bacillus megaterium strain on Lactuca sativa plant growth depending on the origin of the arbuscular mycorrhizal fungus coinoculated: physiologic and biochemical traits. J Plant Growth Regul 7:10–18. https://doi.org/10.1007/s00344-007-9024-5
Flores AC, Luna AAE, Portugal OP (2007) Yield and quality enhancement of marigold flowers by inoculation with Bacillus subtilis and Glomus fasciculatum. J Sustain Agr 31:21–31. https://doi.org/10.1300/J064v31n01_04
Awasthi A, Bharti N, Nair P, Singh R, Shukla A, Gupta M, Darokar M, Kalra A (2011) Synergistic effect of Glomus mosseae and nitrogen fixing Bacillus subtilis strain Daz26 on artemisin content in Artemisia annua L. Appl Soil Ecol 49:125–130. https://doi.org/10.1016/j.apsoil.2011.06.005
Alam M, Khaliq A, Sattar A, Shukla RS, Anwar M, Seema Dharni S (2011) Synergistic effect of arbuscular mycorrhizal fungi and Bacillus subtilis on the biomass and essential oil yield of rose-scented geranium (Pelargonium graveolens). Arch Agron Soil Sci 57:889–898. https://doi.org/10.1080/03650340.2010.498013
Luginbuehl LH, Menard GN, Kurup S, Van Erp H, Radhakrishnan GV, Breakspear A, Oldroyd GED, Eastmond PJ (2017) Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356:1175–1178. https://doi.org/10.1126/science.aam9970
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashra M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32:429–448
Shinde SK, Shinde BP, Patale SW (2013) The alleviation of salt stress by the activity of AM fungi in growth and productivity of onion (Allium cepa l.) plant. Int J Life Sci Pharma Res 3:11–15
Thilagar G, Bagyaraj DJ, Rao MS (2016) Selected microbial consortia developed for chilly reduces application of chemical fertilizers by 50% under field conditions. Sci Hortic 198:27–35. https://doi.org/10.1016/j.scienta.2015.11.021
Romero E, Fernandez-Bayo J, Diaz J, Nogales R (2010) Enzyme activities and diuron persistence in soil amended with vermicompost derived from spent grape marc and treated with urea. Appl Soil Ecol 44:198–204. https://doi.org/10.1016/j.apsoil.2009.12.006
Mengual C, Mauricio S, Azcón R, Roldán A (2014) Microbial inoculants and organic amendment improves plant establishment and soil rehabilitation under semiarid conditions. J Environ Manage 134:1–7. https://doi.org/10.1016/j.jenvman.2014.01.008
Xun F, Xie B, Liu S, Guo C (2015) Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ Sci Pollut Res Int 22(1):598–608. https://doi.org/10.1007/s11356-014-3396-4
Moyano FE, Kutsch WL, Schulze ED (2007) Response of mycorrhizal, rhizosphere and soil basal respiration to temperature and photosynthesis in a barley field. Soil Biol Biochem 39:843–853. https://doi.org/10.1016/j.soilbio.2006.10.001
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54(4):655–670. https://doi.org/10.1046/j.1351-0754.2003.0556.x
Akyol TY, Niwa R, Hirakawa H, Maruyama H, Sato T, Suzuki T, Fukunaga A, Sato T, Yoshida S, Tawaraya K, Saito M, Ezawa T, Sato S (2019) Impact of introduction of arbuscular mycorrhizal fungi on the root microbial community in agricultural fields. Microbes Environ 34(1):23–32. https://doi.org/10.1264/jsme2.ME18109
Selvakumar G, Krishnamoorthy R, Kim K, Sa TM (2016) Genetic diversity and association characters of bacteria isolated from arbuscular mycorrhizal fungal spore walls. PLoS ONE 11:e0160356. https://doi.org/10.1371/journal.pone.0160356
Tsavkelova EA, Cherdyntseva TA, Netrusov AI (2005) Auxin production by bacteria associated with orchid roots. Microbiol 74:46–53. https://doi.org/10.1007/s11021-005-0027-6
Kurm V, van der Putten WH, Pineda A, Hol WHG (2018) Soil microbial species loss affects plant biomass and survival of an introduced bacterial strain, but not inducible plant defences. Ann Bot 121(2):311–319. https://doi.org/10.1093/aob/mcx162
Doongar RC, Rathore AP, Sharma S (2020) Effect of halotolerant plant growth promoting rhizobacteria inoculation on soil microbial community structure and nutrients. Appl Soil Ecol 150:103461. https://doi.org/10.1016/j.apsoil.2019.103461
Qin H, Brookes PC, Xu J (2016) Arbuscular mycorrhizal fungal hyphae alter soil bacterial community and enhance polychlorinated biphenyls dissipation. Front Microbiol 7:939. https://doi.org/10.3389/fmicb.2016.00939
Vik U, Logares R, Rakel B, Rune H, Carlsen T, Ingrid B, Anne-Brit K, Oksta OA, Kauserud H (2013) Different bacterial communities in ectomycorrhizae and surrounding soil. Sci Rep 3:3471. https://doi.org/10.1038/srep03471
Ofek-Lalzar M, Sela N, Goldman-Voronov M (2014) Niche and host-associated functional signatures of the root surface microbiome. Nat Commun 5:4950. https://doi.org/10.1038/ncomms5950
Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014) Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8:1577–1587. https://doi.org/10.1038/ismej.2014.17
Donn S, Kirkegaard JA, Perera G, Richardson AE, Watt M (2015) Evolution of bacterial communities in the wheat crop rhizosphere. Environ Microbiol 17:610–621. https://doi.org/10.1111/1462-2920.12452
Hamonts K, Trivedi P, Garg A, Janitz C, Grinyer J, Holford P, Botha FC, Anderson IC, Singh BK (2018) Field study reveals core plant microbiota and relative importance of their drivers. Environ Microbiol 20(1):124–140. https://doi.org/10.1111/1462-2920.14031
Bhattacharyya D, Duta S, Sang-Mi Yu, Jeong SC, Lee YH (2018) Taxonomic and functional changes of bacterial communities in the rhizosphere of kimchi cabbage after seed bacterization with Proteus vulgaris JBLS202. Plant Pathol J 34(4):286–296. https://doi.org/10.5423/PPJ.OA.03.2018.0047
Barabote RD, Xie G, Leu DH, Normand P, Necsulea A, Daubin V (2009) Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus provides insights into its ecophysiological and evolutionary adaptations. Genome Res 19:1033–1043. https://doi.org/10.1101/gr.084848.108
Xu J, Feng Y, Wang Y, Luo X, Tang J, Lin X (2015) The foliar spray of Rhodopseudomonas palustris grown under stevia residue extract promotes plant growth via changing soil microbial community. J Soil Sediments 65:180–192. https://doi.org/10.1007/s11368-015-1269-1
Pearce DA, Newsham KK, Thorne MAS, Clavo-Bado L, Kresk M, Laskaris P (2012) Metagenomic analysis of a southern mairitime antartic soil. Front Microbiol 3:403. https://doi.org/10.3389/fmicb.2012.00403
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: CS, RD VS and TES. Soil physico-chemical properties and soil microbial activity: VS RD, CS and VJ. Bacterial community analysis: CS, RD TES and MM. CS wrote the draft of the manuscript; RD revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarathambal, C., Dinesh, R., Srinivasan, V. et al. Changes in Bacterial Diversity and Composition in Response to Co-inoculation of Arbuscular Mycorrhizae and Zinc-Solubilizing Bacteria in Turmeric Rhizosphere. Curr Microbiol 79, 4 (2022). https://doi.org/10.1007/s00284-021-02682-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-021-02682-8