Abstract
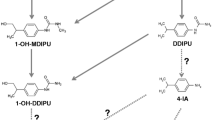
The intense use of pesticides in agricultural activities for the last several decades has caused contamination of the ecosystems connected with crop fields. Despite the well-documented occurrence of pesticide biodegradation by microbes, natural attenuation of atrazine (ATZ), and its effects on ecological processes in subtropical forested areas, such as Iguaçu National Park located in Brazil, has been poorly investigated. Subtropical environments sustain a great degree of fungal biodiversity, and the patterns and roles of these organisms should be better understood. This work aimed to evaluate nine ligninolytic-producer fungi isolated from the INP edge to degrade and detoxify ATZ solutions. ATZ degradation and the main metabolites produced, including deisopropylatrazine and deethylatrazine (DEA), were analyzed using dispersive liquid–liquid microextraction followed by gas chromatography-mass spectrometer. Four fungi were able to degrade ATZ to DEA, and the other five showed potential to grow and facilitate ATZ biodegradation. Furthermore, two strains of Fusarium spp. showed an enhanced potential for detoxification according to the Allium cepa (onion) test. Although the isolates produced ligninolytic enzymes, no ligninolytic activity was observed in the biodegradation of ATZ, a feature with ecological significance. In conclusion, Ascomycota fungi from the INP edge can degrade and detoxify ATZ in solution. Increasing the knowledge of biodiversity in subtropical protected areas, such as ecosystem services provided by microbes, enhances ecosystem conservation.





Similar content being viewed by others
References
Maqbool Z et al (2016) Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environ Sci Pollut Res 23:16904–16925
Moore DR et al (2017) A weight-of-evidence approach for deriving a level of concern for atrazine that is protective of aquatic plant communities. Integr Environ Assess Manag 13(4):686–701
Hansen AM et al (2013) Atrazina: Un herbicida polémico. Rev Int Contam Ambiental 29:65–84
Sousa JCG et al (2018) A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard Mater 344:146–162
WHO (World Health Organization) (2018) A global overview of national regulations and standards for drinking-water quality. WHO, Geneva
NORTOX 500 SC. Prescripción de agrotóxico. Atrazina (2017). http://www.nortox.com.br/wp-content/uploads/2017/05/Atrazina-Nortox-500-SC-BulaVER-04-17.08.2017.pdf. Accessed 20 May 2018
Singh B, Singh K (2016) Microbial degradation of herbicides. Crit Rev Microbiol 42(2):245–261
Hayes TB et al (2002) Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA 99(8):5476–5480
Hirano LQL et al (2019) Effects of egg exposure to atrazine and/or glyphosate on bone development in Podocnemis unifilis (Testudines, Podocnemididae). Ecotoxicol Environ Saf 182:109400
García-Espiñeira M, Tejeda-Benitez L, Olivero-Verbel J (2018) Toxicity of atrazine- and glyphosate-based formulations on Caenorhabditis elegans. Ecotoxicol Environ Saf 156:216–222
Andleeb S et al (2016) Influence of soil pH and temperature on atrazine bioremediation. J Northe Agric Univ 23(2):12–19
Salazar-Ledesma M et al (2018) Mobility of atrazine in soils of a wastewater irrigated maize field. Agr Ecosyst Environ 255:73–83
Koskinen WC, Clay SA (1997) Factors affecting atrazine fate in North Central U.S. soils. Rev Environ Contam Toxicol 151:117–165
Smith AE, Walker A (1989) Prediction of the persistence of the triazine herbicides atrazine, cyanazine, and metribuzin in regina heavy clay. Can J Soil Sci 69:587–595
Kaufman DD, Blake J (1970) Degradation of atrazine by soil fungi. Soil Biol Biochem 2(2):73–80
Singh S et al (2018) Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 16:211–237
Martinez B et al (2001) Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J Bacteriol 183(19):5684–5697
Ma L et al (2017) Rapid biodegradation of atrazine by Ensifer sp. strain and its degradation genes. Int Biodeterior Biodegrad 116:133–140
Yang X et al (2018) Biodegradation of atrazine by the novel Citricoccus sp. strain TT3. Ecotoxicol Environ Saf 147:144–150
Deshmukh R, Khardenavis AA, Purohit HJ (2016) Diverse metabolic capacities of fungi for bioremediation. Indian J Microbiol 56(3):247–264
Fan X, Song F (2014) Bioremediation of atrazine: recent advances and promises. J Soils Sediments 14:1727–1737
Brasil Ministério do Meio Ambiente. Snuc – Sistema Nacional de Unidades de Conservação da Natureza: Lei no. 9.985, de 18 de julho de 2000; Decreto no. 4.340, de 22 de agosto de 2002; Decreto no. 5.746, de 5 de abril de 2006. Plano Estratégico Nacional de Áreas Protegidas: Decreto no. 5.758, de 13 de abril de 2006. Brasília: MMA, 2011. 76 p.
ICMBio – Instituto Chico Mendes de Conservação da Biodiversidade. Parque Nacional do Iguaçu. 2019. Accessed 06 Jun 2019. http://www.icmbio.gov.br/parnaiguacu/.
Salamuni R et al (2002) Parque Nacional do Iguaçu, PR - Cataratas de fama mundial. In: Schobbenhaus C et al (edn) C. Sítios Geológicos e Paleontológicos do Brasil. 1st edn. Brasilia, DNPM/CPRM - Comissão Brasileira de Sítios Geológicos e Paleobiológicos (SIGEP), pp 313–321. http://sigep.cprm.gov.br/sitio011/sitio011.htm. Accessed 2 Jul 2018
Schiesari L et al (2013) Pesticide use and biodiversity conservation in the Amazonian agricultural frontier. Philosoph Trans R Soc B Biol Sci 368:1619
Tedersoo L et al (2014) Global diversity and geography of soil fungi. Science 346:6213
Blackwell M (2011) The fungi: 1, 2, 3 ... 5.1 million species? Am J Bot 98:426–438
Fiskesjo G (1985) The Allium test as a standard in environmental monitoring. Hereditas 102:99–112
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81
Datta S et al (2018) (2018) Assessment of genotoxic effects of pesticide and vermicompost treated soil with Allium cepa test. Sustain Environ Res 28:171–178
Bending GD, Friloux M, Walker A (2002) Degradation of contrasting pesticides by white rot fungi and its relationship with ligninolytic potential. FEMS Microbiol Lett 212(1):59–63
Pereira PM et al (2013) Optimized atrazine degradation by Pleurotus ostreatus INCQS 40310: an alternative for impact reduction of herbicides used in sugarcane crops. J Microb Biochem Technol S12:006
Della-Flora A, et al (2018) Fast, cheap and easy routine quantification method for atrazine and its transformation products in water matrixes using a DLLME-GC/MS method. Anal Methods
Marinho G et al (2017) Potential of the filamentous fungus Aspergillus niger AN 400 to degrade Atrazine in wastewaters. Biocatal Agric Biotechnol 9:162–167
Weber RWS, Pitt D (2000) Teaching techniques for mycology: 11. Riddell’s slide cultures. Mycologist 14(3):118–120
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118
Kumar S, Stecher G, Tamura K (2016) Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16(1):11–120
Australian Government, Review of Atrazine - Technical report: environmental assessment. https://apvma.gov.au/node/14356. Accessed Jan 2018.
Kaufman DD, Kearney PC, Sheets TJ (1965) microbial degradation of simazine microbial. J Agric Food Chem 13(3):238–242
Jeffery S, Burgess LW (1990) Growth of Fusarium graminearum Schwabe group 1 on media amended with atrazine, chlorsulfuron or glyphosate in relation to temperature and osmotic potential. Soil Biol Biochem 22(5):665–670
Oliveira BR et al (2015) Biodegradation of pesticides using fungi species found in the aquatic environment. Environ Sci Pollut Res 22(15):11781–11791
Silveira GL et al (2017) (2017) Chemosphere toxic effects of environmental pollutants: comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178:359–367
Zablotowicz RM, Weaver MA, Locke MA (2006) Microbial adaptation for accelerated atrazine mineralization/degradation in Mississippi Delta soils. Weed Sci 54:538–547
Krutz LJ, Zablotowicz RM, Reddy KN (2012) Selection pressure, cropping system, and rhizosphere proximity affect atrazine degrader populations and activity in s-triazine–adapted soil. Weed Sci 60(3):516–524
Douglass JF, Radosevich M, Tuovinen OH (2017) Microbial attenuation of atrazine in agricultural soils: biometer assays, bacterial taxonomic diversity, and catabolic genes. Chemosphere 176:352–360
Brien HEO et al (2005) Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol 71(9):5544–5550
Waalwijk C et al (1996) Discordant groupings of Fusarium spp. from sections Elegans Liseola and Dlaminia based on ribosomal ITS1 and ITS2sequences. Mycology 88(3):361–368
Srivastava S, Kadooka C, Uchida JY (2018) Fusarium species as pathogen on orchids. Microbiol Res 207:188–195
Tortora GJ, Funke BR, Case CL (2005) Microbiologia, 8a edn. Porto Alegre, Artemed
Janshekar H, Fiechter A (1983) Lignin: biosynthesis, application, and biodegradation. Adv Biochem Eng Biotechnol Berl 27:119–178
Gaur N, Narasimhulu K, Pydisetty Y (2018) Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J Clean Prod 198:1602–1631
Jain R, Garg V, Yadav D (2014) In vitro comparative analysis of monocrotophos degrading potential of Aspergillus flavus,Fusarium pallidoroseum and Macrophomina sp. Biodegradation 25:437–446
Karagianni EP et al (2015) Homologues of xenobiotic metabolizing N-acetyltransferases in plant-associated fungi: novel functions for an old enzyme family. Sci Rep 5:1–14
De Lima DP et al (2018) Fungal bioremediation of pollutant aromatic amines. Curr Opin Green Sustain Chem 11:34–44
Cocaign A et al (2013) Biotransformation of Trichoderma spp. and their tolerance to aromatic amines, a major class of pollutants. Appl Environ Microbiol 79(47):19–26
Tan LR et al (2015) A collection of cytochrome P450 monooxygenase genes involved in modification and detoxification of herbicide atrazine in rice (Oryza sativa) plants. Ecotoxicol Environ Safety 119:25–34
Xing H et al (2014) Effects of atrazine and chlorpyrifos on cytochrome P450 in common carp liver. Chemosphere 104:244–250
Acknowledgements
We want to thank to UNILA for the facilities to develop our projects and all support of DELABEN team members while the execution of this research.
Funding
The research was supported by UNILA University and we received no specific Grant from any funding agency to carry out this work.
Author information
Authors and Affiliations
Contributions
SBE, CKP, MB and RCB planned this research. SBE and MB worked analysis of ATZ degradation and the production of principal metabolites using GC–MS. SBE and RCB worked on morphological characterization, wrote the article and designed figures. GFS made the toxicity evaluation of ATZ and fungi-treated ATZ by Allium cepa test, DCD, WLA and RCB developed isolates’ molecular characterization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Esparza-Naranjo, S.B., da Silva, G.F., Duque-Castaño, D.C. et al. Potential for the Biodegradation of Atrazine Using Leaf Litter Fungi from a Subtropical Protection Area. Curr Microbiol 78, 358–368 (2021). https://doi.org/10.1007/s00284-020-02288-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02288-6