Abstract
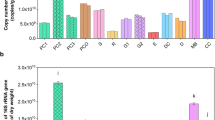
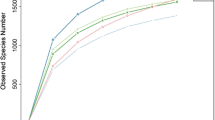
Using black soldier fly (Hermetia illucens) larvae in treatment of livestock manure is a promising technology. In this study, high-throughput sequencing was used to analyze the microbial community in chicken manure before and after treatment with H. illucens larvae. In fresh chicken manure, the most abundant bacterial phylum was Firmicutes (55.58%) followed by Bacteroidetes (24.52%) and then Proteobacteria (12.29%). After treatment of the manure with H. illucens larvae for 15 days, the abundance of Firmicutes increased to 97.72% while that of Bacteroidetes and Proteobacteria decreased. Concomitantly, the most abundant genera of fungi in chicken manure changed from Kernia (46.19%) and Microascus (17.22%) to Penicillium (46.82%) and Aspergillus (45.22%). Correlation-network analysis showed the existence of strong and complex correlations between the dominant operational taxonomic units (OUT) of bacteria and fungi. While most of these correlations were positive, three specific genera, namely g_norank_f_Bacillaceae, Penicillium, and Aspergillus exhibited negative correlations with the remaining genera. These three genera were highly abundant in the intestines of H. illucens and in chicken manure treated with H. illucens larvae. Based on 16S rDNA microbiome-function predictions, the metabolic pathways associated with sugars, amino acids, and organic pollutants inside the intestinal tract of H. illucens were enriched versus those of the other three groups. In summary, the treatment of chicken manure with H. illucens larvae significantly reduced the microbial diversity, while strongly increasing organic metabolism in the intestinal bacteria. This technology shows the potential for applications in livestock manure treatment.





Similar content being viewed by others
References
Wang Q, Awasthi MK, Zhang Z, Wong JWC (2019) Chapter 9—sustainable composting and its environmental implications. In: Taherzadeh MJ, Bolton K, Wong J, Pandey A (eds) Sustainable resource recovery and zero waste approaches. Elsevier, Amsterdam, pp 115–132
Min C, An D, Wang Y, Xu H-H, Wang L-H, Kang M, Wang M-B (2020) Progress of rural solid waste resource utilization in China. J Agric Resour Environ (in Chinese) 37(2):151–160
Kumar K, Gupta SC, Baidoo S, Chander Y, Rosen CJ (2005) Antibiotic uptake by plants from soil fertilized with animal manure. J Environ Qual 34(6):2082–2085
Zhang Y-J, Hu H-W, Gou M, Wang J-T, Chen D, He J-Z (2017) Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ Pollut 231:1621–1632
Pratt C, Redding M, Hill J, Jensen PD (2015) Does manure management affect the latent greenhouse gas emitting potential of livestock manures? Waste Manag 46:568–576
Bernal M, Alburquerque J, Moral R (2009) Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol 100(22):5444–5453
DA Neher WT, Bates ST, Leff JW, Fierer N (2013) Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 8(11):e79512
Sheppard DC, Newton GL, Thompson SA, Savage S (1994) A value added manure management system using the black soldier fly. Bioresour Technol 50(3):275–279
Miller BF, Teotia JS, Thatcher TO (1974) Digestion of poultry manure by Musca domestica. Br Poult Sci 15(2):231–234
Salomone R, Saija G, Mondello G, Giannetto A, Fasulo S, Savastano D (2017) Environmental impact of food waste bioconversion by insects: application of life cycle assessment to process using Hermetia illucens. J Clean Prod 140:890–905
Li Q, Zheng L, Cai H, Garza E, Yu Z, Zhou S (2011) From organic waste to biodiesel: black soldier fly, Hermetia illucens, makes it feasible. Fuel 90(4):1545–1548
Bondari K, Sheppard D (1987) Soldier fly, Hermetia illucens L., larvae as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquac Res 18(3):209–220
Kawasaki K, Hashimoto Y, Hori A, Kawasaki T, Hirayasu H, Iwase S-i, Hashizume A, Ido A, Miura C, Miura T, Nakamura S, Seyama T, Matsumoto Y, Kasai K, Fujitani Y (2019) Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals 9(3):98
Choi Y-C, Choi J-Y, Kim J-G, Kim M-S, Kim W-T, Park K-H, Bae S-W, Jeong G-S (2009) Potential usage of food waste as a natural fertilizer after digestion by Hermetia illucens (Diptera: Stratiomyidae). Int J Ind Entomol 19(1):171–174
Akinmutimi A, Amaechi C (2015) Comparative effects of poultry manure, piggery manure and npk fertilizer on the growth, yield and nutrient content of okra (Abelmoschus esculentus). Int J Curr Res 7(12):1–6
Newton G, Sheppard D, Watson D, Burtle G, Dove C, Tomberlin J, Thelen E (2005) The black soldier fly, Hermetia illucens, as a manure management/resource recovery tool. In: Symposium on the state of the science of Animal Manure and Waste Management. p 5–7
Beskin KV, Holcomb CD, Cammack JA, Crippen TL, Knap AH, Sweet ST, Tomberlin JK (2018) Larval digestion of different manure types by the black soldier fly (Diptera: Stratiomyidae) impacts associated volatile emissions. Waste Manag 74:213–220
Sheppard C (1983) House fly and lesser fly control utilizing the black soldier fly in manure management systems for caged laying hens. Environ Entomol 12(5):1439–1442
Bradley SW, Sheppard D (1984) House fly oviposition inhibition by larvae of Hermetia illucens, the black soldier fly. J Chem Ecol 10(6):853–859
Liu Q, Tomberlin JK, Brady JA, Sanford MR, Yu Z (2008) Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Environ Entomol 37(6):1525–1530
Erickson MC, Islam M, Sheppard C, Liao J, Doyle MP (2004) Reduction of Escherichia coli O157: H7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. J Food Prot 67(4):685–690
Kim W, Bae S, Park K, Lee S, Choi Y, Han S, Koh Y (2011) Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J Asia-Pac Entomol 14(1):11–14
Vogel H, Müller A, Heckel DG, Gutzeit H, Vilcinskas A (2018) Nutritional immunology: diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev Comp Immunol 78:141–148
Jeon H, Park S, Choi J, Jeong G, Lee S-B, Choi Y, Lee S-J (2011) The intestinal bacterial community in the food waste-reducing larvae of Hermetia illucens. Curr Microbiol 62(5):1390–1399
Zheng L, Crippen TL, Singh B, Tarone AM, Dowd S, Yu Z, Wood TK, Tomberlin JK (2013) A survey of bacterial diversity from successive life stages of black soldier fly (Diptera: Stratiomyidae) by using 16S rDNA pyrosequencing. J Med Entomol 50(3):647–658
Jiang CL, Jin WZ, Tao XH, Zhang Q, Zhu J, Feng SY, Xu XH, Li HY, Wang ZH, Zhang ZJ (2019) Black soldier fly larvae (Hermetia illucens) strengthen the metabolic function of food waste biodegradation by gut microbiome. Microb Biotechnol 12(3):528–543
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821
Gu W, Lu Y, Tan Z, Xu P, Xie K, Li X, Sun L (2017) Fungi diversity from different depths and times in chicken manure waste static aerobic composting. Bioresour Technol 239:447–453
Bonelli M, Bruno D, Caccia S, Sgambetterra G, Cappellozza S, Jucker C, Tettamanti G, Casartelli M (2019) Structural and functional characterization of Hermetia illucens larval midgut. Front Physiol 10:204
Park SI, Kim JW, Yoe SM (2015) Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev Comp Immunol 52(1):98–106
Park SI, Chang BS, Yoe SM (2014) Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol Res 44(2):58–64
Bruno D, Bonelli M, De Filippis F, Di Lelio I, Tettamanti G, Casartelli M, Ercolini D, Caccia S (2019) The intestinal microbiota of Hermetia illucens larvae is affected by diet and shows a diverse composition in the different midgut regions. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01864-18
Varotto BI, Ottoboni M, Martin E, Comandatore F, Vallone L, Spranghers T, Eeckhout M, Mereghetti V, Pinotti L, Epis S (2017) A survey of the mycobiota associated with larvae of the black soldier fly (Hermetia illucens) reared for feed production. PLoS ONE 12(8):e0182533
Wang H, Sangwan N, Li H-Y, Su J-Q, Oyang W-Y, Zhang Z-J, Gilbert JA, Zhu Y-G, Ping F, Zhang H-L (2017) The antibiotic resistome of swine manure is significantly altered by association with the Musca domestica larvae gut microbiome. ISME J 11(1):100
Barson G, Renn N, Bywater AF (1994) Laboratory evaluation of six species of entomopathogenic fungi for the control of the house fly (Musca domestica L.), a pest of intensive animal units. J Invertebr Pathol 64(2):107–113
Mishra S, Kumar P, Malik A, Satya S (2011) Adulticidal and larvicidal activity of Beauveria bassiana and Metarhizium anisopliae against housefly, Musca domestica (Diptera: Muscidae), in laboratory and simulated field bioassays. Parasitol Res 108(6):1483–1492
Sharififard M, Mossadegh M, Vazirianzadeh B, Zarei-Mahmoudabadi A (2011) Interactions between Entomopathogenic fungus, Metarhizium anisopliae and sublethal doses of spinosad for control of house fly, Musca domestica. Iran J Arthropod-Borne Dis 5(1):28
Malik A, Singh N, Satya S (2007) House fly (Musca domestica): a review of control strategies for a challenging pest. J Environ Sci Health B 42(4):453–469
Lam K, Tsang M, Labrie A, Gries R, Gries G (2010) Semiochemical-mediated oviposition avoidance by female house flies, Musca domestica, on animal feces colonized with harmful fungi. J Chem Ecol 36(2):141–147
Lam K, Thu K, Tsang M, Moore M, Gries G (2009) Bacteria on housefly eggs, Musca domestica, suppress fungal growth in chicken manure through nutrient depletion or antifungal metabolites. Naturwissenschaften 96(9):1127–1132
Larrondo Veliz J, Calvo M (1990) The antimicrobial capacity of Penicillium and Aspergillus strains isolated from vineyard soils. Microb Lett 44(174):77–81
Bergel F, Morrison AL, Moss AR, Klein R, Rinderknecht H, Ward JL (1943) An antibacterial substance from Aspergillus clavatus and Penicillium claviforme and its probable identity with patulin. Nature 152(3869):750
Miranda CD, Cammack JA, Tomberlin JK (2019) Interspecific competition between the house fly, Musca domestica L. (Diptera: Muscidae) and black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae) when reared on poultry manure. Insects 10(12):440
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant No. 2018YFD0510402), Major Science and Technology Innovation Project of Shandong Province (No. 2017GGH5129), Key Research and Development project of Shandong Province (No. 2019GSF107094), Natural Science Foundation of Shandong Province (ZR2016YL031), Innovation Team Project for Modern Agricultural Industrious Technology System of Shandong Province (SDAIT-11-10), Project of Shandong Province Higher Educational Science and Technology Program (No. J18KB077), and Yantai Science and Technology Project (No. 2017NC049).
Author information
Authors and Affiliations
Contributions
XZ, GC and ZF designed the research; JZ, JZ and XZ performed the experiments; LJ, XY, HZ and GC analyzed data and contributed figures and tables; GC wrote the article; and ZZ advised on the manuscript content and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Research Involving Human and/or Animals Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Zhang, J., Jiang, L. et al. Black Soldier Fly (Hermetia illucens) Larvae Significantly Change the Microbial Community in Chicken Manure. Curr Microbiol 78, 303–315 (2021). https://doi.org/10.1007/s00284-020-02276-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02276-w