Abstract
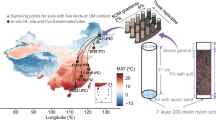
Glacier retreat may result in the decomposition of old organic carbon stored at the frontier of glacier retreat and the release of greenhouse gases such as CO2 and methane into the atmosphere. This process may gradually transform the soil in the region from its original status as a carbon sink into a carbon source, thus producing a positive feedback effect on global warming. In this study, Laohugou Glacier No. 12, Qilian Mountains, China, was taken as the research object, and the newly melted soil (Q1) at the frontier of glacier retreat and the sandy soil (Q2) on the bank of the nearby river were collected. The content of accumulation of organic matter (AOM) in Q1 soil was 5.56 ± 0.27 g/kg, and the total nitrogen was 0.60 ± 0.03 g/kg, which was significantly higher than that in Q2. The soil microbial carbon metabolism of Q2 was significantly (P < 0.01) higher than that of Q1 and the ability of organic matter to decompose was greater. The alpha diversity index of bacteria, fungi and archaea of Q2 was significantly higher than that of Q1. It may be that there were dominant species in Q1 causing the lower species evenness. The archaea metabolic function genes in Q1 were higher than those in Q2 because archaea are better adapted to a frozen environment. Bacterial carbohydrate and amino acid metabolism was abundant in Q2 and was related to microbial transformation of the carbon source into CO2.




Similar content being viewed by others
References
Siles JA, Margesin R (2017) Seasonal soil microbial responses are limited to changes in functionality at two Alpine forest sites differing in altitude and vegetation. Sci Rep 7:2204
Zhu F, Wang S, Zhou PJ (2003) Flavobacterium xinjiangense sp. nov. and Flavobacterium omnivorum sp. nov., novel psychrophiles from the China No. 1 glacier. Int J Syst Evol Microbiol 53:853–857
Zhang YS, Liu SY, Shangguan DH, Li J, Zhao JD (2012) Thinning and Shrinkage of Laohugou No. 12 Glacier in the Western Qilian Mountains, China, from 1957 to 2007. J Mt Sci 9:343–350
Bolch T (2007) Climate change and glacier retreat in northern Tien Shan (Kazakhstan/Kyrgyzstan) using remote sensing data. Glob Planet Change 56:1–12
Oerlemans J (1994) Quantifying global warming from the retreat of glaciers. Science 264:243–245
Zhang LN, Jiang Y, Zhao SD, Jiao L, Wen Y (2018) Relationships between tree age and climate sensitivity of radial growth in different drought conditions of Qilian mountains, Northwestern China. Forests 9:135
Chen JZ, Kang SC, Qin X, Du WT, Sun WJ, Liu YS (2017) The mass-balance characteristics and sensitivities to climate variables of Laohugou Glacier No. 12, western Qilian Mountains, China. Sci Cold Arid Regions 9:0543–0553
Liu YS, Qin X, Chen JZ, Li ZL, Wang J, Du WT, Guo WQ (2018) Variations of Laohugou Glacier No. 12 in the western Qilian Mountains, China, from 1957 to 2015. J Mt Sci 15:25–32
Zhang SH, Hou SG, Qin X, Du WT, Liang F, Li ZG (2015) Preliminary study on effects of glacial retreat on the dominant glacial snow bacteria in Laohugou Glacier No. 12. Geomicrobiol J 32:113–118
Kapur B, Pasquale S, Tekin S, Todorovic M, Sezen SM, Özfidaner M, Gümüs Z (2010) Prediction of climatic change for the next 100 years in Southern Italy. Sci Res Essays 5:1470–1478
Wilhelm L, Singer GA, Fasching C, Battin TJ, Besemer K (2013) Microbial biodiversity in glacier-fed streams. ISME J 7:1651–1660
Mayor JR et al (2017) Elevation alters ecosystem properties across temperate treelines globally. Nature 542:91–95
Zhang SH, Yang GL, Wang YT, Hou SG (2010) Abundance and community of snow bacteria from three glaciers in the Tibetan Plateau. J Environ Sci 22:1418–1424
Heijden MGAVD, Bardgett RD, Straalen NMV (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Foght J, Aislabie J, Turner S, Brown CE, Ryburn J, Saul DJ, Lawson W (2004) Culturable bacteria in subglacial sediments and ice from two Southern Hemisphere glaciers. Microb Ecol 47:329–340
Sigler WV, Crivii S, Zeyer J (2002) Bacterial succession in glacial forefield soils characterized by community structure, activity and opportunistic growth dynamics. Microb Ecol 44:306–316
Tscherko D, Rustemeier J, Richter A, Wanek W, Kandeler E (2003) Functional diversity of the soil microflora in primary succession across two glacier forelands in the Central Alps. Eur J Soil Sci 54:685–696
Wietrzyk P, Rola K, Osyczka P, Nicia P, Szymański W, Węgrzyn M (2018) The relationships between soil chemical properties and vegetation succession in the aspect of changes of distance from the glacier forehead and time elapsed after glacier retreat in the Irenebreen foreland (NW Svalbard). Plant Soil 428:195–211
Zou JW, Rogers WE, DeWalt SJ, Siemann E (2006) The effect of Chinese tallow tree (Sapium sebiferum) ecotype on soil-plant system carbon and nitrogen processes. Oecologia 150:272–281
Oorts K, Vanlauwe B, Merckx R (2003) Cation exchange capacities of soil organic matter fractions in a Ferric Lixisol with different organic matter inputs. Agric Ecosyst Environ 100:161–171
Parfitt RL, Giltrap DJ, Whitton JS (1995) Contribution of organic matter and clay minerals to the cation exchange capacity of soils. Commun Soil Sci Plan 26:1343–1355
Krogh L, Breuning-Madsen H, Greve MH (2000) Cation-exchange capacity pedotransfer functions for danish soils. Acta Agric Scand 50:1–12
Ghiri MN, Rezaei M, Sameni A (2012) Zinc sorption–desorption by sand, silt and clay fractions in calcareous soils of Iran. Arch Agron Soil Sci 58:945–957
Prietzel J, Dümig A, Wu YH, Zhou J, Klysubun W (2013) Synchrotron-based P K-edge XANES spectroscopy reveals rapid changes of phosphorus speciation in the topsoil of two glacier foreland chronosequences. Geochim Cosmochim Acta 108:154–171
Sharpley AN (1985) The selective erosion of plant nutrients in runoff. Soil Sci Soc Am J 49:1527–1534
Mcisaac GF, Hirschi MC, Mitchell JK (1991) Nitrogen and phosphorus in eroded sediment from corn and soybean tillage systems. J Environ Qual 20:663–670
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359
Freeman C, Ostle NJ, Fenner N, Kang H (2004) A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol Biochem 36:1663–1667
Choi KH, Dobbs FC (1999) Comparison of two kinds of Biolog microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J Microbiol Meth 36:203–213
Kim JS, Sparovek G, Longo RM, Melo WJD, Crowley D (2007) Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol Biochem 39:684–690
Cui XQ, Ren JW, Qin X, Sun WJ, Yu GM, Wang ZB, Liu WG (2014) Chemical characteristics and environmental records of a snow-pit at the Glacier No. 12 in the Laohugou Valley, Qilian Mountains. J Earth Sci 25:379–385
Galand PE, Casamayor EO, Kirchman DL, Lovejoy C (2009) Ecology of the rare microbial biosphere of the Arctic Ocean. Proc Natl Acad Sci USA 106:22427–22432
Ciccarelli FD, Doerks T, Mering CV, Creevey CJ, Snel B, Bork P (2006) Toward automatic reconstruction of a highly resolved tree of life. Science 311:1283–1287
Dion P (2008) Extreme views on prokaryote evolution. In: Dion P, Nautiyal CS (eds) Microbiology of extreme soils. Springer, Berlin, Heidelberg, pp 45–70
Liu JB et al (2016) Diversity and succession of autotrophic microbial community in high-elevation soils along deglaciation chronosequence. FEMS Microbiol Ecol 92:160
Strauss SL, Garcia-Pichel F, Day TA (2012) Soil microbial carbon and nitrogen transformations at a glacial foreland on Anvers Island, Antarctic Peninsula. Polar Biol 35:1459–1471
Bowman JP, McCammon SA, Brown MV, Nichols DS, McMeekin TA (1997) Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl Environ Microbiol 63:3068–3078
Romano I, Giordano A, Lama L, Nicolaus B, Gambacorta A (2005) Halomonas campaniensis sp. nov., a haloalkaliphilic bacterium isolated from a mineral pool of Campania Region, Italy. Syst Appl Microbiol 28:610–618
Jørgensen BB, Cohen Y, Revsbech NP (1986) Transition from anoxygenic to oxygenic photosynthesis in a Microcoleus chthonoplastes cyanobacterial mat. Appl Environ Microbiol 51:408–417
Zeng J et al (2016) Primary succession of nitrogen cycling microbial communities along the deglaciated forelands of Tianshan Mountain, China. Front Microbiol 7:1353
Hu CX, Liu YD (2003) Primary succession of algal community structure in desert soil. Acta Bot Sin 45:917–924
Takeuchi N, Uetake J, Fujita K, Aizen VB, Nikitin SD (2006) A snow algal community on Akkem Glacier in the Russian Altai Mountains. Ann Glaciol 43:378–384
Péter G, Rosa C (2006) Biodiversity and ecophysiology of yeasts. Springer, Berlin, Heidelberg
Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Zumsteg A et al (2012) Bacterial, Archaeal and fungal succession in the forefield of a receding glacier. Microb Ecol 63:552–564
Tsuji M, Uetake J, Tanabe Y (2016) Changes in the fungal community of Austre Brøggerbreen deglaciation area, Ny-Ålesund, Svalbard, High Arctic. Mycoscience 57:448–451
Olsen V, Hyatt AD, Boyle DG, Mendez D (2004) Co-localisation of Batrachochytrium dendrobatidis and keratin for enhanced diagnosis of chytridiomycosis in frogs. Dis Aquat Org 61:85–88
Nicol GW, Tscherko D, Embley TM, Prosser JI (2005) Primary succession of soil Crenarchaeota across a receding glacier foreland. Environ Microbiol 7:337–347
Walker CB et al (2010) Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107:8818–8823
Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 51708340 and 41971073), Key Research and Development Program of Shandong Province, P.R. China (No. 2019GSF109103), International Postdoctoral Exchange Fellowship Program (No. 20180063), Special Financial Grant from the China Postdoctoral Science Foundation (No. 2015T80738), and National Major Science and Technology Program for Water Pollution Control and Treatment (No. 2017ZX07101001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhang, Y., Chen, H. et al. Soil Properties and Microbial Diversity at the Frontier of Laohugou Glacier Retreat in Qilian Mountains. Curr Microbiol 77, 425–433 (2020). https://doi.org/10.1007/s00284-019-01846-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01846-x