Abstract
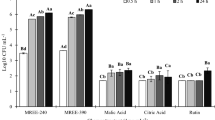
Bacillus velezensis strain S3-1 has a broad range of hosts and is used as a biocontrol agent and biofertilizer. However, the interaction of maize root exudates and colonization of the strain S3-1 has not yet been investigated. In our study, strain S3-1 effectively colonized both rhizosphere soil and root tissue. Collected maize root exudates significantly induced the chemotaxis, cluster movement, and biofilm formation of strain S3-1, showing increases of 1.43, 1.6, and 2.08 times, respectively, compared with the control. In addition, the components of root exudates (organic acids: citric acid, malic acid, and oxalic acid; amino acids: glycine, proline and phenylalanine; sugars: glucose, fructose, and sucrose) were tested. Each of these compounds could induce chemotactic response, swarming motility, and biofilm formation significantly. The strongest chemotactic response and swarming motility were found when malic acid was applied, but maximal ability of biofilm formation was stimulated by proline. Furthermore, we found that these compounds of root exudates stimulated the population of S3-1 adhering to the maize root surface, especially in the presence of malic acid. These results indicate that maize root exudates play an important role in the colonization of S3-1, and provide a deeper understanding of the interaction between plants and microorganisms.





Similar content being viewed by others
References
Agrios G (2004) Plant pathology, 5th edn. Academic Press, Cambridge, pp 339–342
Alejandro J, Esther M, José DF, Paula G, Pedro FM, Raúl R (2016) Effective colonization of spinach root surface by Rhizobium. BMC Genom 16:685–690
Almoneafy A, Kakar K, Nawaz Z, Li B, Yang C, Xie G (2014) Tomato plant growth promotion and antibacterial related-mechanisms of four rhizobacterial Bacillus strains against Ralstonia solanacearum. Symbiosis 63:59–70
Ai X (2016) Effect of Bacillus amyloliquefaciens S3-1 on diseases control and growth-promotion of tomato and the separation of its active ingredients. Master dissertation, Shanghai Normal University, Shanghai (in Chinese with English abstract)
Anang D, Rusul G, Radu S, Bakar J, Beuchat L (2006) Inhibitory effect of oxalic acid on bacterial spoilage of raw chilled chicken. J Food Prot 69:1913–1919
Baudoin E, Benizri E, Guckert A (2003) Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biol Biochem 35:1183–1192
Beauregard P, Chai Y, Vlamakis H, Losick R, Kolter R (2013) Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci USA 110:1621–1630
Beneduzi A, Ambrosini A, Passaglia L (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Bhardwaj D, Ansari M, Sahoo R, Tuteja N (2014) Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact 13:66–77
Bradley D (1980) A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol 26:146–154
Chen Y, Cao S, Chai Y, Clardy J, Kolter R, Guo J, Losick R (2012) A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol 85:418–430
Chen X, Zhang Y, Fu X, Li Y, Wang Q (2016) Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biol Technol 115:113–121
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
de Weert S, Vermeiren H, Mulders I, Kuiper I, Hendrickx N, Bloemberg G, Vanderleyden J, De Mot R, Lugtenberg B (2002) Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant Microbe Interact 15:1173–1180
Fang R, Jia Lin, Yao S, Wang Y, Wang J, Zhou C, Wang H, Xiao M (2013) Promotion of plant growth, biological control and induced systemic resistance in maize by Pseudomonas aurantiaca JD37. Ann Microbiol 63:1137–1185
Fan B, Carvalhais L, Becker A, Fedoseyenko D, von Wirén N, Borriss R (2012) Transcriptomic profiling of Bacillus amyloliquefaciens FZB42 in response to maize root exudates. BMC Microbiol 12:116–130
Gaworzewska E, Carlile M (1982) Positive chemotaxis of Rhizobium leguminosarum and other bacteria towards root exudates from legumes and other plants. Microbiology 128:1179–1188
Hao W, Ren L, Ran W, Shen Q (2010) Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 336:485–497
Kamilova F, Kravchenko L, Shaposhnikov A, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact 19:250–256
Kloepper J, Schroth M, Miller T (1980) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70:1078–1082
Köhler T, Curty L, Barja F, Van Delden C, Pechère J (2000) Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182:5990–5996
Li S, Xu C, Wang J, Guo B, Yang L, Chen J, Ding W (2017) Cinnamic, myristic and fumaric acids in tobacco root exudates induce the infection of plants by Ralstonia solanacearum. Plant Soil 412:381–395
Ling N, Raza W, Ma J, Huang Q, Shen Q (2011) Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol 47:374–379
Mwita L, Chan W, Pretorius T, Lyantagaye S, Lapa S, Avdeeva L, Reva O (2016) Gene expression regulation in the plant growth promoting Bacillus atrophaeus UCMB-5137 stimulated by maize root exudates. Gene 590:18–28
O’Brien PA (2017) Biological control of plant diseases. Australas Plant Pathol 46:293–304
Park S, Kim R, Ryu C, Choi S, Lee C, Kim J, Park S (2008) Citrinin, a mycotoxin from Penicillium citrinum, plays a role in inducing motility of Paenibacillus polymyxa. FEMS Microbiol Ecol 65:229–237
Saleem M, Arshad M, Hussain S, Bhatti A (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Sang M, Kim K (2014) Biocontrol activity and root colonization by Pseudomonas corrugata strains CCR26 and CCR26 against Phytophthora blight of pepper. Biocontrol 59:437–448
Shi S, Richardson A, O’Callaghan M, DeAngelis K, Jones E, Stewart A, Firestone MK, Condron L (2011) Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol Ecol 77:600–610
Si F (2014) Selection and application of a high-resistance strain with its study of resistance mechanism. Master dissertation, Shanghai Normal University, Shanghai (in Chinese with English abstract)
Suo B, Chen Q, Wu W, Wu D, Tian M, Jie Y, Zhang B, Wen J (2016) Chemotactic responses of Phytophthora sojae zoospores to amino acids and sugars in root exudates. J Chen Plant Pathol 82:142–148
Taguchi K, Fukutomi H, Kuroda A, Kato J, Ohtake H (1997) Genetic identification of chemotactic transducers for amino acids in Pseudomonas aeruginosa. Microbiology 143:3223–3229
Tan S, Yang C, Mei X, Shen S, Wasee R, Shen Q, Xu Y (2013) The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl Soil Ecol 64:15–22
Van de Broek A, Lambrecht M, Vanderleyden J (1998) Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense. Microbiology 144:2599–2606
Weng J, Wang Y, Li J, Shen Q, Zhan R (2013) Enhanced root colonization and biocontrol activity of Bacillus amyloliquefaciens SQR9 by abrB gene disruption. Appl Microbiol Biotechnol 97:8823–8830
Wu K, Su L, Fang Z, Yuan S, Wang L, Shen B, Shen Q (2017) Competitive use of root exudates by Bacillus amyloliquefaciens with Ralstonia solanacearum decreases the pathogenic population density and effectively controls tomato bacterial wilt. Sci Hortic 218:132–138
Wu K, Yuan S, Xun G, Shi W, Pan B, Guan H, Shen B, Shen Q (2015) Root exudates from two tobacco cultivars affect colonization of Ralstonia solanacearum and the disease index. Eur J Plant Pathol 141:667–677
Yuan J, Zhang N, Huang Q, Raza W, Li R, Vivanco J, Shen Q (2015) Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Sci Rep 5:13438–13447
Zhang F, Meng X, Yang X, Ran W, Shen Q (2014) Quantification and role of organic acids in cucumber root exudates in Trichoderma harzianum T-E5 colonization. Plant Physiol Biochem 83:250–257
Zhang N, Wang D, Liu Y, Li S, Shen Q, Zhang R (2014) Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 374:689–700
Zhang N, Yang D, Wang D, Miao Y, Shao J, Zhou X, Xu Z, Li Q, Feng H, Li S, Shen Q, Zhang R (2015) Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genom 16:685–706
Zuo C, Li C, Li B, Wei Y, Hu C, Yang Q, Yang J, Sheng O, Kuang R, Deng G, Biswas M (2015) The toxic mechanism and bioactive components of Chinese leek root exudates acting against Fusarium oxysporum f. sp. cubense tropical race 4. Eur J Plant Pathol 143:447–460
Acknowledgements
This work was supported by Shanghai Municipal Science and Technology Commission (Grant No. 16391902100), the Foundation of Key Laboratory of Urban Agriculture (Grant No. UA201705), Ministry of Agriculture of the People’s Republic of China and Shanghai Engineering and Technical Research Center of Plant Germplasm Resources (Grant No. 17DZ2252700), and the National Key R&D Program of China (Grant No. 2016YFC0502702).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, Y., Zhu, H., Luo, S. et al. Role of Maize Root Exudates in Promotion of Colonization of Bacillus velezensis Strain S3-1 in Rhizosphere Soil and Root Tissue. Curr Microbiol 76, 855–862 (2019). https://doi.org/10.1007/s00284-019-01699-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01699-4