Abstract
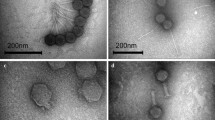
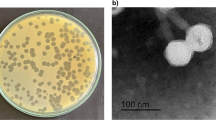
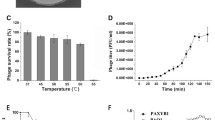
In this study, two lytic phages designated as ϕPSZ1 and ϕPSZ2 infecting multidrug resistant Pseudomonas aeruginosa were isolated from sewage samples collected in Zagazig, Egypt. Morphological analysis by transmission electron microscopy revealed that both phages belong to the podoviridae family and resembles typical T7-like phages. ϕPSZ1 has a head of about 60 ± 5 nm in diameter with a short tail of 19 ± 2 nm in length, while ϕPSZ2 has a head of about 57 ± 5 nm in diameter with a short tail of 14 ± 2 nm in length. Both phages were shown to be able to infect 13 different P. aeruginosa strains and has no effect on other tested bacteria. In spite of morphological similarity, these phages showed diverged genomic sequences revealed by restriction enzyme digestion analysis. One-step growth curves of bacteriophages revealed eclipse and latent periods of 12 min for ϕPSZ1 and 15 min for ϕPSZ2, respectively, with burst sizes of about 100 per infected cell. Phage treatment prevented the growth of P. aeruginosa for up to 18 h with multiplicity of infection ratios of 1. These results suggest that both phages have a high potential for phage application to control P. aeruginosa.





Similar content being viewed by others
References
Abdel-Haliem MEF, Askora A (2013) Isolation and characterization of bacteriophages of Helicobacter pylori isolated from Egypt. Future Virol 8:821–826
Ackermann HW (2000) Basic phage electron microscopy. Methods Mol Biol 501:113–126
Ackermann HW (2009) Phage classification and characterization. Methods Mol Cell Biol 501:127–140
Adams MH (1959) Bacteriophages. Interscience Publishers, New York
Bru¨ssow H (2005) Phage therapy: the Escherichia coli experience. Microbiology 151:2133–2140
Bruttin A, Brussow H (2005) Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 49:2874–2878
Ceyssens PJ, Lavigne R (2010) Bacteriophages of Pseudomonas. Future Microbiol 5:1041–1055
Drenkard E (2003) Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect 5:1213–1219
Driscoll JA, Brody SL, Kollef MH (2007) The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67:351–368
Giske CG, Monnet DL, Cars O, Carmeli Y (2008) Clinical and economic impact of common multidrug-resistant Gram-negative bacilli. Antimicrob Agents Chemother 52:813–821
Greer GG (2005) Bacteriophage control of foodborne bacteria. J Food Prot 68:1102–1111
Jones JB, Jackson LE, Balogh B, Obradovic A, Iriarte FB, Momol MT (2007) Bacteriophages for plant disease control. Annu Rev Phytopathol 45:245–262
Kerr KG, Snelling AM (2009) Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect 73:338–344
Lambert PA (2002) Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med 95:22–26
Lavigne R, Burkal’tseva MV, Robben J, Sykilinda NN, Kurochkina LP, Grymonprez B, Jonckx B, Krylov VN, Mesyanzhinov VV, Volckaerta G (2003) The genome of bacteriophage φKMV, a T7-like virus infecting Pseudomonas aeruginosa. Virology 31(2):49–59
Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GAA (2003) Genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310
Matar GM, Chaar MH, Araj GF, Srour Z, Jamaleddine G, Hadi U (2005) Detection of a highly prevalent and potentially virulent strain of Pseudomonas aeruginosa from nosocomial infections in a medical center. BMC Microbiol 5:29–34
McVay CS, Velasquez M, Fralick JA (2007) Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother 51:1934–1938
Merabishvili M, Pirnay JP, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V et al (2009) Quality-controlled small-scale production of a well defined bacteriophage cocktail for use in human clinical trials. PLoS One 4:e4944
Merabishvili M, Verhelst R, Glonti T, Chanishvili N, Krylov V, Cuvelier C, Tediashvili M, Vaneechoutte M (2007) Digitized fluorescent RFLP analysis (RFLP) as a universal method for comparing genomes of culturable dsDNA viruses: application to bacteriophages. Res Microbiol 158:572–581
Merril CR, Scholl D, Adhya S (2006) Phage therapy. In: Calendar R (ed) The bacteriophages. Oxford University Press, New York, pp 725–739
Nakai T, Park SC (2002) Bacteriophage therapy of infectious diseases in aquaculture. Res Microbiol 153:13–18
Pajunen M, Kiljunen S, Skurnik M et al (2000) Bacteriophage phi YeO3-12, specific for Yersinia enterocolitica serotype O: 3, is related to coliphages T3 and T7. J Bacteriol 182:5114–5120
Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A (2009) Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 18:237–238
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358(358):135–138
Sulakvelidze A (2005) Phage therapy: an attractive option for dealing with antibiotic-resistant bacterial infections. Drug Discov Today 10:807–809
Veesenmeyer JL, Hauser AR, Lisboa T, Rello J (2009) Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med 37:1777–1786
Wagenaar JA, Bergen MAPV, Mueller MA, Wassenaar TM, Carlton RM (2005) Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet Microbiol 109:275–283
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Didamony, G.E., Askora, A. & Shehata, A.A. Isolation and Characterization of T7-Like Lytic Bacteriophages Infecting Multidrug Resistant Pseudomonas aeruginosa Isolated from Egypt. Curr Microbiol 70, 786–791 (2015). https://doi.org/10.1007/s00284-015-0788-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-015-0788-8