Abstract
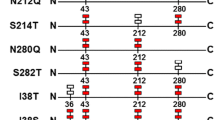
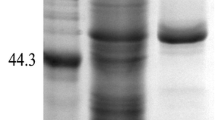
Many studies have demonstrated that the properties of enzymes expressed in eukaryotes can be affected by the position and extent of glycosylation on enzyme. In this study, two potential glycosylation sites (the 8th and the 58th asparagine) were identified and the effect of propeptide glycosylation on Rhizomucor miehei lipase (RML) expressed in Pichia pastoris was investigated. To better understand the effect of glycosylation on the activity of RML, three mutants (M1, generated by N8A; M2, generated by N58A; and M3, generated by N8A and N58A) were designed to generate deglycosylated enzymes. The results showed that deglycosylated RML exhibited a twofold higher activity compared to the wild type. However, it was also found that glycosylation on the propeptide was important for the removal of the propeptide by Kex2 protease and secretion of the enzyme. Thus, our study provided a further understanding into the role of glycosylation on enzyme function.






Similar content being viewed by others
References
Aertgeerts K, Ye S, Shi L, Prasad SG, Witmer D, Chi E, Sang BC, Wijnands RA, Webb DR, Swanson RV (2009) N-linked glycosylation of dipeptidyl peptidase IV (CD26): effects on enzyme activity, homodimer formation, and adenosine deaminase binding. Protein Sci 13(1):145–154
Baker D, Sohl JL, Agard DA (1992) A protein-folding reaction under kinetic control. Nature 356(6366):263–265
Bobrowicz P, Davidson RC, Li H, Potgieter TI, Nett JH, Hamilton SR, Stadheim TA, Miele RG, Bobrowicz B, Mitchell T (2004) Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: production of complex humanized glycoproteins with terminal galactose. Glycobiology 14(9):757–766
Boel E, Huge-Jensen B, Christensen M, Thim L, Fiil NP (1988) Rhizomucor miehei triglyceride lipase is synthesized as a precursor. Lipids 23(7):701–706
Brenner C, Fuller RS (1992) Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc Natl Acad Sci 89(3):922–926
Chen YJ, Inouye M (2008) The intramolecular chaperone-mediated protein folding. Curr Opin Struct Biol 18(6):765–770
Eder J, Rheinnecker M, Fersht AR (1993) Folding of subtilisin BPN’: characterization of a folding intermediate. Biochemistry 32(1):18–26
Han ZL, Han SY, Zheng SP, Lin Y (2009) Enhancing thermostability of a Rhizomucor miehei lipase by engineering a disulfide bond and displaying on the yeast cell surface. Appl Microbiol Biotechnol 85(1):117–126
Huge-Jensen B, Andreasen F, Christensen T, Christensen M, Thim L, Boel E (1989) Rhizomucor miehei triglyceride lipase is processed and secreted from transformed Aspergillus oryzae. Lipids 24(9):781–785
Huge-Jensen B, Galluzzo DR, Jensen RG (1987) Partial purification and characterization of free and immobilized lipases from Mucor miehei. Lipids 22(8):559–565
Kukuruzinska M, Bergh M, Jackson B (1987) Protein glycosylation in yeast. Annu Rev Biochem 56(1):915–944
Lis H, Sharon N (2005) Protein glycosylation. Eur J Biochem 218(1):1–27
Nagai K, Ihara Y, Wada Y, Taniguchi N (1997) N-glycosylation is requisite for the enzyme activity and Golgi retention of N-acetylglucosaminyltransferase III. Glycobiology 7(6):769–776
Petrescu A-J, Milac A-L, Petrescu SM, Dwek RA, Wormald MR (2004) Statistical analysis of the protein environment of N-glycosylation sites: implications for occupancy, structure, and folding. Glycobiology 14(2):103–114
Rodrigues RC, Fernandez-Lafuente R (2010) Lipase from Rhizomucor miehei as an industrial biocatalyst in chemical process. J Mol Catal B 64(1):1–22
Seitz O (2000) Glycopeptide synthesis and the effects of glycosylation on protein structure and activity. ChemBioChem 1(4):214–246
Shinde U, Inouye M (1995) Folding pathway mediated by an intramolecular chaperone: characterization of the structural changes in pro-subtilisin E coincident with autoprocessing. J Mol Biol 252(1):25–30
Sinclair AM, Elliott S (2005) Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci 94(8):1626–1635
Tang SJ, Shaw JF, Sun KH, Sun GH, Chang TY, Lin CK, Lo YC, Lee GC (2001) Recombinant expression and characterization of the Candida rugosa lip4 lipase in Pichia pastoris: comparison of glycosylation, activity, and stability. Arch Biochem Biophys 387(1):93–98
Wang J, Wang D, Wang B, Mei Z-h, Liu J, Yu H-W (2012) Enhanced activity of Rhizomucor miehei lipase by directed evolution with simultaneous evolution of the propeptide. Appl Microbiol Biotechnol 96(2):443–450
Winther JR, Sørensen P (1991) Propeptide of carboxypeptidase Y provides a chaperone-like function as well as inhibition of the enzymatic activity. Proc Natl Acad Sci 88(20):9330–9334
Zhang WG, Han SY, Wei DZ, Lin Y, Wang XN (2008) Functional display of Rhizomucor miehei lipase on surface of Saccharomyces cerevisiae with higher activity and its practical properties. J Chem Technol Biotechnol 83(3):329–335
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (21176215/21176102) and Outstanding Young Scholar of Zhejiang Province (R4110092) and the Program for Zhejiang Leading Team of S&T Innovation (2011R50007). We are grateful for the editors and reviewers and thank all the members of Prof. Yu’group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Xie, W. & Yu, H. Enhanced Activity of Rhizomucor miehei Lipase by Deglycosylation of Its Propeptide in Pichia pastoris . Curr Microbiol 68, 186–191 (2014). https://doi.org/10.1007/s00284-013-0460-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-013-0460-0