Abstract
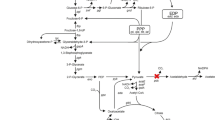
The Gluconobacter oxydans M5 with disruption of the pyrroloquinoline quinine-dependent membrane-bound aldehyde dehydrogenase (ALDH) was used for the oxidation of benzyl alcohol. The selectivity toward benzaldehyde showed an obvious increase for the engineered strain, which reached the 67.3%, while the wild strain had only 2.8%. Meantime, the aqueous/isooctane (1:1) biphasic system was used for the further improvement of selectivity. By these methods, nearly 100% selectivity and conversion rate could be obtained within 1 h at the optimum initial benzyl alcohol concentration of 5.0 g/l.





Similar content being viewed by others
References
Pina DC, Falletta E, Rossi M (2008) Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by bimetallic gold–copper catalyst. J Catal 260:384–386
Jia AZ, Lou LL, Zhang C, Zhang YQ, Liu SX (2009) Selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide over alkali-treated ZSM-5 zeolite catalysts. J Mol Catal A Chem 306:123–129
Caravati M, Grunwaldt JD, Baiker A (2004) Selective oxidation of benzyl alcohol to benzaldehyde in “supercritical” carbon dioxide. Catal Today 91–92:1–5
Bijudas K, Nair TDR (2004) Selective oxidation of benzyl alcohol with monochromate in non-polar solvents. Indian J Chem A 43:1216–1218
Schrader J, Etschmann MM, Sell D, Hilmer JM (2004) Applied biocatalysis for the synthesis of natural flavour compounds current industrial processes and future prospects. Biotechnol Lett 26:463–472
De Muynck C, Pereira CS, Naessens M, Parmentier S, Soetaert W, Vandamme EJ (2007) The genus Gluconobacter oxydans: comprehensive overview of biochemistry and biotechnological applications. Crit Rev Biotechnol 27:147–171
Deppenmeier U, Hoffmeister M, Prust C (2002) Biochemistry and biotechnological applications of Gluconobacter strains. Appl Microbiol Biotechnol 60:233–242
Gupta A, Singh VK, Qazi QN, Kumar A (2001) Gluconobacter oxydans: its biotechnological applications. J Mol Microbiol Biotechnol 3:445–456
Celik D, Bayraktar E, Mehmetoglu D (2004) Biotransformation of 2-phenylethanol to phenylacetaldehyde in a two-phase fed-batch system. Biochem Eng J 17:5–13
Karra-Chaabouni M, Pulvin S, Meziani A, Thomas D, Touraud D, Kunz W (2003) Biooxidation of n-hexanol by alcohol oxidase and catalase in biphasic and micellar systems without solvent. Biotechnol Bioeng 81:27–32
Gandolfi R, Cavenago K, Gualandris R, Gago JVC, Molinari F (2004) Production of 2-phenylacetic acid and phenylacetaldehyde by oxidation of 2-phenylethanol with free immobilized cells of Acetobacter aceti. Process Biochem 39:747–751
Gandolfi R, Ferrara N, Molinari F (2001) An easy and efficient method for the production of carboxylic acids and aldehydes by microbial oxidation of primary alcohols. Tetrahedron Lett 42:513–514
Molinari F, Aragozzini F, Leon R, Prazeres DMF, Gandolfi R (1999) Biotransformations in two-liquid-phase systems—production of phenylacetaldehyde by oxidation of 2-phenylethanol with acetic acid bacteria. Enzyme Microb Technol 25:729–735
Villa R, Romano A, Gandolfi R, Gago JVS, Molinari F (2002) Chemoselective oxidation of primary alcohols to aldehydes with Gluconobacter oxydans. Tetrahedron Lett 43:6059–6061
Fukaya M, Tayama K, Tamaki T, Tagami H (1989) Cloning of the membrane-bound aldehyde dehydrogenase gene of Acetobacter polyoxogenes and improvement of acetic acid production by use of the cloned gene. Appl Environ Microbiol 55:171–176
Fukaya M, Tayama K, Okumura H, Kawamura Y, Beppu T (1989) Purification and characterization of membrane-bound aldehyde dehydrogenase from Acetobacter polyoxogenes sp. Appl Microbiol Biotechnol 32:176–180
Asakura A, Hoshino T (1999) Gluconobacter alcohol/aldehyde dehydrogenase. United States Patent 5932463
Yang XP, Wei LJ, Lin JP, Yin B, Wei DZ (2008) Membrane-bound pyrroloquinoline quinone-dependent dehydrogenase in Gluconobacter oxydans M5, responsible for production of 6-(2-hydroxyethyl) amino-6-deoxy-l-sorbose. Appl Environ Microbiol 74:5250–5253
Wei LJ (2009) Study on the two membrane-bound dehydrogenases of Gluconobacter oxydans, alcohol dehydrogenase and acetaldehyde dehydrogenase, which involved in the oxidation of 1, 2-propanediol to d-(-) lactic acid. Ph.D. thesis, East China University of Science and Technology, Shanghai
Prust C, Hoffmeister M, Liesegang H, Wiezer A, Fricke WF (2005) Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat Biotechnol 23:195–200
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791
Matsushita K, Fujii Y, Ano Y et al (2003) 5-Keto-d-gluconate production is catalyzed by a quinoprotein glycerol dehydrogenase, major polyol dehydrogenase, in Gluconobacter species. Appl Environ Microbiol 69:1959–1966
Matsushita K, Toyama H, Adachi O (1994) Respiratory chains and bioenergetics of acetic acid bacteria. Adv Microb Physiol 36:247–301
Su W, Chang ZY, Gao KL, Wei DZ (2004) Enantioselective oxidation of racemic 1,2-propanediol to d-(-)-lactic acid by Gluconobacter oxydans. Tetrahedron Asymmetry 15:1275–1277
Matsushita K, Toyama H, Adachi O, Miyoshi H (1999) The quinohemoprotein alcohol dehydrogenase of Gluconobacter suboxydans has ubiquinol oxidation activity at a site different from the ubiquinone reduction site. Biochim Biophys Acta Bioenerg 1409:154–164
Islami MSA, Saifi-Abolhassan M, Sepehr Sh, Soudi MR, Mossavi-Nejad SZ (2008) Purification and characterization of alcohol dehydrogenase from Gluconobacter suboxydans. Pak J Biol Sci 11:208–213
Schweiger P, Volland S, Deppenmeier U (2007) Overproduction and characterization of two distinct aldehyde-oxidizing enzymes from Gluconobacter oxydans 621H. J Mol Microbiol Biotechnol 13:147–155
Acknowledgments
This work was financially supported by the National Key Basic Research Development Program of China (“973” Program, No.2009CB724703), and the National Special Fund for State Key Laboratory of Bioreactor Engineering, Grant No. 2060204.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, J., Li, M.H., Lin, J.P. et al. Highly Selective Oxidation of Benzyl Alcohol Using Engineered Gluconobacter Oxydans in Biphasic System. Curr Microbiol 62, 1123–1127 (2011). https://doi.org/10.1007/s00284-010-9831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9831-y