Abstract
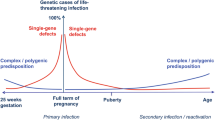
The burden of newborn infectious disease has long been recognized as the highest across the entire human life span. The precise underlying cause is unfortunately still far from clear. A substantial body of data derived mostly from in vitro experimentation indicates “lower” host immune responses in early vs. adult life and is briefly summarized within this review. However, emerging data derived mostly from in vivo experimentation reveal that the newborn host also exhibits an exuberant immune and inflammatory response following infection when compared to the adult. In this context, it is important to emphasize that “infection” does not equate “infectious disease,” as for many infections it is the host response to the infection that causes disease. This simple insight readily arranges existing evidence into cause-effect relationships that explain much of the increase in clinical suffering from infection in early life. We here briefly summarize the evidence in support of this paradigm and highlight the important implications it has for efforts to ameliorate the suffering and dying from infection in early life.

Similar content being viewed by others
References
Scully JL (2004) What is a disease? EMBO Rep 5:650–653
Soares MP, Teixeira L, Moita LF (2017) Disease tolerance and immunity in host protection against infection. Nature reviews 17:83–96
Liu L et al (2012) Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161
Shane AL, Sanchez PJ, Stoll BJ (2017) Neonatal sepsis. Lancet
Wynn JL (2016) Defining neonatal sepsis. Curr Opin Pediatr 28:135–140
Cantey JB, Wozniak PS, Sanchez PJ (2015) Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J 34:267–272
Hornik CP et al (2012) Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr Infect Dis J 31:799–802. https://doi.org/10.1097/INF.1090b1013e318256905c
Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O (2017) Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46:350–363
Zhang X, Zhivaki D, Lo-Man R (2017) Unique aspects of the perinatal immune system. Nature reviews
Ulas T et al (2017) S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat Immunol 18:622–632
Wynn JL, Neu J, Moldawer LL, Levy O (2009) Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol: official journal of the California Perinatal Association 29:79–88
Marshall JC (2014) Why have clinical trials in sepsis failed? Trends Mol Med 20:195–203
Dictionary, Vol. 2017 (2017)
Lewis DB et al (2006) Newborn immunology: relevance to the clinician. Current Problems in Pediatric and Adolescent Health Care 36:189–204
Basha S, Surendran N, Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10:1171–1184
Wang G et al (2010) “Default” generation of neonatal regulatory T cells. J Immunol 185:71–78
Rueda CM et al (2015) Neonatal regulatory T cells have reduced capacity to suppress dendritic cell function. Eur J Immunol 45:2582–2592
Echeverry A, Saijo S, Schesser K, Adkins B (2010) Yersinia enterocolitica promotes robust mucosal inflammatory T cell immunity in murine neonates. Infect Immun 78:3595–3608
Evans IA, Jones CA (2005) HSV induces an early primary Th1 CD4 T cell response in neonatal mice, but reduced CTL activity at the time of the peak adult response. Eur J Immunol 35:1454–1462
Adkins B, Jones M, Bu Y, Levy RB (2004) Neonatal tolerance revisited again: specific CTL priming in mouse neonates exposed to small numbers of semi- or fully allogeneic spleen cells. Eur J Immunol 34:1901–1909
Huygens A et al (2015) Functional exhaustion limits CD4+ and CD8+ T cell responses to congenital cytomegalovirus infection. J Infect Dis 212:484–494. https://doi.org/10.1093/infdis/jiv1071
Mascart F et al (2003) Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J Immunol 170:1504–1509
Marchant A et al (2003) Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest 111:1747–1755
Ota MO et al (2004) Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine 22:511–519
Siegrist CA, Aspinall R (2009) B cell responses to vaccination at the extremes of age. Nature reviews 9:185–194
Halsey N, Galazka A (1985) The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull World Health Organ 63:1151–1169
Rodwell RL, Taylor KM, Tudehope DI, Gray PH (1993) Hematologic scoring system in early diagnosis of sepsis in neutropenic newborns. Pediatr Infect Dis J 12:372–376
Engle WA, McGuire WA, Schreiner RL, Yu PL (1988) Neutrophil storage pool depletion in neonates with sepsis and neutropenia. J Pediatr 113:747–749
Cuenca AG et al (2015) Delayed emergency myelopoiesis following polymicrobial sepsis in neonates. Innate Immun 21:386–391. https://doi.org/10.1177/1753425914542445
Gentile LF et al (2015) Improved emergency myelopoiesis and survival in neonatal sepsis by caspase-1/11 ablation. Immunology 145:300–311. https://doi.org/10.1111/imm.12450
Schelonka RL, Yoder BA, desJardins SE, Hall RB, Butler J (1994) Peripheral leukocyte count and leukocyte indexes in healthy newborn term infants. J Pediatr 125:603–606
Carr R (2000) Neutrophil production and function in newborn infants. Br J Haematol 110:18–28
Levy O et al (1999) Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 104:1327–1333
Prosser A et al (2013) Phagocytosis of neonatal pathogens by peripheral blood neutrophils and monocytes from newborn preterm and term infants. Pediatr Res 74:503–510
Yost CC et al (2009) Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 113:6419–6427
Byrd AS et al (2016) NETosis in neonates: evidence of a reactive oxygen species-independent pathway in response to fungal challenge. J Infect Dis 213:634–639
Hallwirth U, Pomberger G, Pollak A, Roth E, Spittler A (2004) Monocyte switch in neonates: high phagocytic capacity and low HLA-DR expression in VLBWI are inverted during gestational aging. Pediatr Allergy Immunol 15:513–516
Filias A et al (2011) Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr 11:29
Marodi L et al (1994) Candidacidal mechanisms in the human neonate. Impaired IFN-gamma activation of macrophages in newborn infants. J Immunol 153:5643–5649
Gille C, Spring B, Tewes L, Poets CF, Orlikowsky T (2006) A new method to quantify phagocytosis and intracellular degradation using green fluorescent protein-labeled Escherichia coli: comparison of cord blood macrophages and peripheral blood macrophages of healthy adults. Cytometry A 69:152–154
Velilla PA, Rugeles MT, Chougnet CA (2006) Defective antigen-presenting cell function in human neonates. Clin Immunol 121:251–259
Klein RB et al (1977) Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics 60:467–472
Canaday DH et al (2006) Class II MHC antigen presentation defect in neonatal monocytes is not correlated with decreased MHC-II expression. Cell Immunol 243:96–106
Chheda S, Palkowetz KH, Garofalo R, Rassin DK, Goldman AS (1996) Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-alpha and its receptors. Pediatr Res 40:475–483
Kollmann TR et al (2009) Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 183:7150–7160
Mathias B et al (2017) LPS stimulation of cord blood reveals a newborn-specific neutrophil transcriptomic response and cytokine production. Shock 47:606–614
Wynn JL et al (2008) Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112:1750–1758
Kollmann TR, Levy O, Montgomery RR, Goriely S (2012) Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37:771–783
Corbett NP et al (2010) Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 5:e15041
Angelone DF et al (2006) Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-α production in vitro and in vivo. Pediatr Res 60:205–209
Wong OH, Huang F-P, Chiang AKS (2005) Differential responses of cord and adult blood-derived dendritic cells to dying cells. Immunology 116:13–20
Della Chiesa M et al (2014) Human NK cell response to pathogens. Semin Immunol 26:152–160
Lee YC, Lin SJ (2013) Neonatal natural killer cell function: relevance to antiviral immune defense. Clinical & Developmental Immunology 2013:427696
Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C (2011) Natural killer cell responses to infections in early life. Journal of Innate Immunity 3:280–288
D'Andrea A et al (2000) Neonatal invariant Valpha24+ NKT lymphocytes are activated memory cells. Eur J Immunol 30:1544–1550
Yagupsky P, Nolte FS (1990) Quantitative aspects of septicemia. Clin Microbiol Rev 3:269–279
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16:343–353. https://doi.org/10.1038/ni.3123
Medzhitov R, Schneider DS, Soares MP (2012) Disease tolerance as a defense strategy. Science 335:936–941
Zhao J et al (2008) Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci U S A 105:7528–7533
Cuenca AG et al (2015) TRIF-dependent innate immune activation is critical for survival to neonatal gram-negative sepsis. J Immunol 194:1169–1177. https://doi.org/10.4049/jimmunol.1302676
Sugitharini V, Prema A, Berla Thangam E (2013) Inflammatory mediators of systemic inflammation in neonatal sepsis. Inflammation Research: Official Journal of the European Histamine Research Society 62:1025–1034
Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA (2010) The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol 185:2980–2988
Dupont A et al (2016) Age-dependent susceptibility to enteropathogenic Escherichia coli (EPEC) infection in mice. PLoS Pathog 12:e1005616
Byun H-J et al (2007) An evaluation of the neonatal immune system using a Listeria infection model. Neonatology 92:83–90
Kronforst KD et al (2012) A neonatal model of intravenous Staphylococcus epidermidis infection in mice < 24 h old enables characterization of early innate immune responses. PLoS One 7:e43897
Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA (2012) Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol 42:311–319
Seok J et al (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A
Rittirsch D, Flierl MA, Ward PA (2008) Harmful molecular mechanisms in sepsis. Nature reviews 8:776–787
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5
Vogl T et al (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13:1042–1049
Vogl T, Gharibyan AL, Morozova-Roche LA (2012) Pro-inflammatory S100A8 and S100A9 proteins: self-assembly into multifunctional native and amyloid complexes. Int J Mol Sci 13:2893–2917
Foell D, Frosch M, Sorg C, Roth J (2004) Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clinica chimica acta; international journal of clinical chemistry 344:37–51
Viemann D et al (2005) Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 105:2955–2962
Foell D, Wittkowski H, Vogl T, Roth J (2007) S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 81:28–37
Foell D, Wittkowski H, Roth J (2009) Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 58:859–868
Austermann J et al (2014) Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflammatory conditions. Cell Rep 9:2112–2123
West MA, Heagy W (2002) Endotoxin tolerance: a review. Crit Care Med 30:S64–S73
Biswas SK, Lopez-Collazo E (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30:475–487
Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M (2014) Immune cells in term and preterm labor. Cellular & Molecular Immunology 11:571–581
Sharma AA, Jen R, Butler A, Lavoie PM (2012) The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol 145:61–68. https://doi.org/10.1016/j.clim.2012.1008.1006
Heinemann AS et al (2017) In neonates S100A8/S100A9 alarmins prevent the expansion of a specific inflammatory monocyte population promoting septic shock. FASEB J 31:1153–1164
Yamamoto M et al (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643
Weighardt H et al (2006) Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J Immunol 177:5623–5630
Kanagavelu S et al (2015) TIR domain-containing adapter-inducing beta interferon (TRIF) mediates immunological memory against bacterial pathogens. Infect Immun 83:4404–4415
Kolb JP, Casella CR, SenGupta S, Chilton PM, Mitchell TC (2014) Type I interferon signaling contributes to the bias that toll-like receptor 4 exhibits for signaling mediated by the adaptor protein TRIF. Sci Signal 7:ra108
Wynn JL et al (2015) Postnatal age is a critical determinant of the neonatal host response to sepsis. Molecular Medicine (Cambridge, Mass) 21:496–504
Vogl T, Leukert N, Barczyk K, Strupat K, Roth J (2006) Biophysical characterization of S100A8 and S100A9 in the absence and presence of bivalent cations. Biochim Biophys Acta 1763:1298–1306
Austermann J, Zenker S, Roth J (2017) S100-alarmins: potential therapeutic targets for arthritis. Expert Opin Ther Targets 21:739–751
Tanimura N et al (2014) The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int Immunol 26:307–314
Cuenca, A.G. et al. Critical role for CXC ligand 10/CXC receptor 3 signaling in the murine neonatal response to sepsis. Infect Immun. 79, 2746–2754. doi: https://doi.org/10.1128/IAI.01291-01210. (2011)
Amenyogbe N, Kollmann TR, Ben-Othman R (2017) Early-life host-microbiome interphase: the key frontier for immune development. Frontiers in pediatrics 5:111
Gensollen T, Iyer SS, Kasper DL, Blumberg RS (2016) How colonization by microbiota in early life shapes the immune system. Science 352:539–544
Belkaid Y, Harrison OJ (2017) Homeostatic immunity and the microbiota. Immunity 46:562–576
Strunk T, Kollmann T, Patole S (2015) Probiotics to prevent early-life infection. Lancet Infect Dis 15:378–379. https://doi.org/10.1016/S1473-3099(1015)70088-70085
Underwood MA (2017) Impact of probiotics on necrotizing enterocolitis. Semin Perinatol 41:41–51
Panigrahi P et al (2017) A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature
Neu J, Walker WA (2011) Necrotizing enterocolitis. N Engl J Med 364:255–264
Nino DF, Sodhi CP, Hackam DJ (2016) Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 13:590–600
Adkins B, Leclerc C, Marshall-Clarke S (2004) Neonatal adaptive immunity comes of age. Nature Reviews 4:553–564
Gentile LF et al (2014) Protective immunity and defects in the neonatal and elderly immune response to sepsis. J Immunol 192:3156-3165. https://doi.org/10.4049/jimmunol.1301726
Levy O et al (2006) The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol 177:1956–1966
Lee S et al (2013) Genetic determinants for cadmium and arsenic resistance among Listeria monocytogenes serotype 4b isolates from sporadic human listeriosis patients. Appl Environ Microbiol 79:2471–2476
Funding information
This research was supported by a Michael Smith Foundation for Health Research Career Investigator Award to T.R.K. This work was supported by grants from the Appenrodt Foundation, the German Research Foundation (VI 538/6-1), and the Volkswagen Foundation (Az 90005) to DV.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Immunocompetence of the Newborn - Guest Editors: Arnaud Marchant and Tobias Kollmann
Rights and permissions
About this article
Cite this article
Brook, B., Harbeson, D., Ben-Othman, R. et al. Newborn susceptibility to infection vs. disease depends on complex in vivo interactions of host and pathogen. Semin Immunopathol 39, 615–625 (2017). https://doi.org/10.1007/s00281-017-0651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-017-0651-z