Abstract
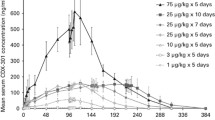
Purpose: The recombinant human interleukin-1 receptor (rhu IL-1R) is a soluble truncated form of the type 1 full-length membrane-bound receptor that binds IL-1 with identical affinity to that of the membrane form. As such, it may have clinical potential by sequestering IL-1, thereby preventing it from binding to its membrane-bound receptor and eliciting a biological effect. As IL-1 has been shown to regulate leukemic cell proliferation in an autocrine fashion, a phase I trial of rhu IL-1R was conducted in patients with relapsed and refractory acute myeloid leukemia (AML). Methods: The study group comprised 11 patients who were sequentially treated on one of three dose levels, receiving a single intravenous (i.v.) bolus dose on day 1 followed by 13 days of daily subcutaneous (s.c.) injections with the option of an additional 14 days of treatment if a response of stable disease or better was achieved. Dose level 1 i.v. bolus 500 g/m2, s.c. dose 250 g/m2 per day (five patients); dose level 2 i.v. bolus 1000 g/m2, s.c. dose 500 g/m2 per day (three patients); dose level 3 i.v. bolus 2000 g/m2, s.c. dose 1000 g/m2 per day (three patients). Owing to limited drug availability, the study was designed to only examine these three dose levels. Results: rhu IL-1R was well tolerated. There was no grade 3 or 4 non-hematological toxicity related to the study drug and the maximum tolerated dose was not reached. No IL-1R-blocking antibodies developed during the course of the study. Serum levels of IL-1, IL-6 and TNF were undetectable before, during and after rhu IL-1R administration. The terminal half-life after i.v. dosing was at least 7–12 h, and after s.c. dosing 2–4 days. Serum levels of rhu IL-1R up to 360- and 25-fold those of pretreatment levels were achieved after i.v. and s.c. dosing respectively. No patient had a complete, partial or minor response to treatment; four had stable disease and seven had progressive disease. Conclusions: rhu IL-1R therapy was safe but did not have any apparent antileukemic effect at the doses administered.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 6 October 1997 / Accepted: 1 April 1998
Rights and permissions
About this article
Cite this article
Bernstein, S., Fay, J., Frankel, S. et al. A phase I study of recombinant human soluble interleukin-1 receptor (rhu IL-1R) in patients with relapsed and refractory acute myeloid leukemia. Cancer Chemother Pharmacol 43, 141–144 (1999). https://doi.org/10.1007/s002800050874
Issue Date:
DOI: https://doi.org/10.1007/s002800050874