Abstract
Purpose
Arginine depletion interferes with pyrimidine metabolism as well as DNA damage repair pathways. Preclinical data indicates that pairing pegylated arginine deiminase (ADI-PEG 20) with fluoropyrimidines or platinum enhances cytotoxicity in vitro and in vivo in arginine auxotrophs.
Methods
This is a single-center, open-label, phase 1 trial of ADI-PEG 20 and modified FOLFOX6 (mFOLFOX6) in treatment-refractory hepatocellular carcinoma (HCC) and other advanced gastrointestinal tumors. A 3 + 3 dose escalation design was employed to assess safety, tolerability, and determine the recommended phase 2 dose (RP2D) of ADI-PEG 20. A RP2D expansion cohort for patients with HCC was employed to define the objective response rate (ORR). Secondary objectives were to estimate progression-free survival (PFS), overall survival (OS), and to explore pharmacodynamics and immunogenicity. Eligible patients were treated with mFOLFOX6 intravenously biweekly at standard doses and ADI-PEG-20 intramuscularly weekly at 18 (Cohort 1) or 36 mg/m2 (Cohort 2 and RP2D expansion).
Results
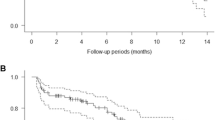
Twenty-seven patients enrolled—23 with advanced HCC and 4 with other gastrointestinal tumors. No dose-limiting toxicities were observed in cohort 1 or 2. The RP2D for ADI-PEG 20 was 36 mg/m2 weekly with mFOLFOX6. The most common any grade adverse events (AEs) were thrombocytopenia, neutropenia, leukopenia, anemia, and fatigue. Among the 23 HCC patients, the most frequent treatment-related Grade ≥ 3 AEs were neutropenia (47.8%), thrombocytopenia (34.7%), leukopenia (21.7%), anemia (21.7%), and lymphopenia (17.4%). The ORR for this group was 21% (95% CI 7.5–43.7). Median PFS and OS were 7.3 and 14.5 months, respectively. Arginine levels were depleted with therapy despite the emergence of low levels of anti-ADI-PEG 20 antibodies. Arginine depletion at 4 and 8 weeks and archival tumoral argininosuccinate synthetase-1 levels did not correlate with response.
Conclusions
Concurrent mFOLFOX6 plus ADI-PEG-20 intramuscularly at 36 mg/m2 weekly shows an acceptable safety profile and favorable efficacy compared to historic controls. Further evaluation of this combination is warranted in advanced HCC patients.



Similar content being viewed by others
References
Patil MD, Bhaumik J, Babykutty S, Banerjee UC, Fukumura D (2016) Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene 35(38):4957–4972. https://doi.org/10.1038/onc.2016.37
Murray PJ (2016) Amino acid auxotrophy as a system of immunological control nodes. Nat Immunol 17(2):132–139. https://doi.org/10.1038/ni.3323
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP (2017) Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 14(1):11–31. https://doi.org/10.1038/nrclinonc.2016.60
Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA (2002) Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res 62(19):5443–5450
Kremer JC, Prudner BC, Lange SES, Bean GR, Schultze MB, Brashears CB, Radyk MD, Redlich N, Tzeng SC, Kami K, Shelton L, Li A, Morgan Z, Bomalaski JS, Tsukamoto T, McConathy J, Michel LS, Held JM, Van Tine BA (2017) Arginine deprivation inhibits the Warburg effect and upregulates glutamine anaplerosis and serine biosynthesis in ASS1-deficient cancers. Cell Rep 18(4):991–1004. https://doi.org/10.1016/j.celrep.2016.12.077
Changou CA, Chen YR, Xing L, Yen Y, Chuang FY, Cheng RH, Bold RJ, Ann DK, Kung HJ (2014) Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proc Natl Acad Sci USA 111(39):14147–14152. https://doi.org/10.1073/pnas.1404171111
Miraki-Moud F, Ghazaly E, Ariza-McNaughton L, Hodby KA, Clear A, Anjos-Afonso F, Liapis K, Grantham M, Sohrabi F, Cavenagh J, Bomalaski JS, Gribben JG, Szlosarek PW, Bonnet D, Taussig DC (2015) Arginine deprivation using pegylated arginine deiminase has activity against primary acute myeloid leukemia cells in vivo. Blood 125(26):4060–4068. https://doi.org/10.1182/blood-2014-10-608133
Harding JJ, Abou-Alfa GK (2014) Treating advanced hepatocellular carcinoma: How to get out of first gear. Cancer 120(20):3122–3130. https://doi.org/10.1002/cncr.28850
Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De Rosa V, Cremona F, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA, Ng C, Curley SA (2004) Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 22(10):1815–1822. https://doi.org/10.1200/JCO.2004.11.120
Glazer ES, Piccirillo M, Albino V, Di Giacomo R, Palaia R, Mastro AA, Beneduce G, Castello G, De Rosa V, Petrillo A, Ascierto PA, Curley SA, Izzo F (2010) Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol 28(13):2220–2226. https://doi.org/10.1200/JCO.2009.26.7765
Yang TS, Lu SN, Chao Y, Sheen IS, Lin CC, Wang TE, Chen SC, Wang JH, Liao LY, Thomson JA, Wang-Peng J, Chen PJ, Chen LT (2010) A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer 103(7):954–960. https://doi.org/10.1038/sj.bjc.6605856
Szlosarek PW, Steele JP, Nolan L, Gilligan D, Taylor P, Spicer J, Lind M, Mitra S, Shamash J, Phillips MM, Luong P, Payne S, Hillman P, Ellis S, Szyszko T, Dancey G, Butcher L, Beck S, Avril NE, Thomson J, Johnston A, Tomsa M, Lawrence C, Schmid P, Crook T, Wu BW, Bomalaski JS, Lemoine N, Sheaff MT, Rudd RM, Fennell D, Hackshaw A (2017) Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol 3(1):58–66. https://doi.org/10.1001/jamaoncol.2016.3049
Abou-Alfa GK, Qin S, Ryoo B-Y, Lu S-N, Yen C-J, Feng Y-H, Lim HY, Izzo F, Colombo M, Sarker D, Bolondi L, Vaccaro GM, Harris WP, Chen Z, Hubner R, Meyer T, Bomalaski JS, Lin C, Chao Y, Chen L-T (2018) Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 29(6):1402–1408
Rabinovich S, Adler L, Yizhak K, Sarver A, Silberman A, Agron S, Stettner N, Sun Q, Brandis A, Helbling D, Korman S, Itzkovitz S, Dimmock D, Ulitsky I, Nagamani SC, Ruppin E, Erez A (2015) Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 527(7578):379–383. https://doi.org/10.1038/nature15529
Thongkum A, Wu C, Li YY, Wangpaichitr M, Navasumrit P, Parnlob V, Sricharunrat T, Bhudhisawasdi V, Ruchirawat M, Savaraj N (2017) The combination of arginine deprivation and 5-fluorouracil improves therapeutic efficacy in argininosuccinate synthetase negative hepatocellular carcinoma. Int J Mol Sci. https://doi.org/10.3390/ijms18061175
McAlpine JA, Lu HT, Wu KC, Knowles SK, Thomson JA (2014) Down-regulation of argininosuccinate synthetase is associated with cisplatin resistance in hepatocellular carcinoma cell lines: implications for PEGylated arginine deiminase combination therapy. BMC Cancer 14:621. https://doi.org/10.1186/1471-2407-14-621
Savaraj N, Wu C, Li YY, Wangpaichitr M, You M, Bomalaski J, He W, Kuo MT, Feun LG (2015) Targeting argininosuccinate synthetase negative melanomas using combination of arginine degrading enzyme and cisplatin. Oncotarget 6(8):6295–6309. https://doi.org/10.18632/oncotarget.3370
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G, Investigators R (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66. https://doi.org/10.1016/S0140-6736(16)32453-9
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. https://doi.org/10.1056/NEJMoa0708857
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
Cheng A-L, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, Baron AD, Park J-W, Han G, Jassem J, Blanc J-F, Vogel A, Komov D, Evans TRJ, López-López C, Dutcus CE, Ren M, Kraljevic S, Tamai T, Kudo M (2017) Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 35(15_suppl):4001–4001. https://doi.org/10.1200/JCO.2017.35.15_suppl.4001
Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y, Lee JH, Sun Y (2013) Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 31(28):3501–3508. https://doi.org/10.1200/JCO.2012.44.5643
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Beddowes E, Spicer J, Chan PY, Khadeir R, Corbacho JG, Repana D, Steele JP, Schmid P, Szyszko T, Cook G, Diaz M, Feng X, Johnston A, Thomson J, Sheaff M, Wu BW, Bomalaski J, Pacey S, Szlosarek PW (2017) Phase 1 dose-escalation study of pegylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1-deficient thoracic cancers. J Clin Oncol 35(16):1778–1785. https://doi.org/10.1200/JCO.2016.71.3230
Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ (2006) Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 24(25):4085–4091. https://doi.org/10.1200/JCO.2006.06.9039
Lowery MA, Yu KH, Kelsen DP, Harding JJ, Bomalaski JS, Glassman DC, Covington CM, Brenner R, Hollywood E, Barba A, Johnston A, Liu KC, Feng X, Capanu M, Abou-Alfa GK, O’Reilly EM (2017) A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer 123(23):4556–4565. https://doi.org/10.1002/cncr.30897
Abou-Alfa GK, Meyer T, Cheng A-L, El-Khoueiry AB, Rimassa L, Ryoo B-Y, Cicin I, Merle P, Park J-W, Blanc J-F, Bolondi L, Klümpen HJ, Chan SL, Dadduzio V, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK (2018) Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol 36(4_suppl):207. https://doi.org/10.1200/JCO.2018.36.4_suppl.207
Zhu AX, Kang Y-K, Yen C-J, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Lim HY, Pracht M, Rau K-M, Merle P, Motomura K, Ohno I, Daniele B, Shin D, Gerken G, Abada P, Hsu Y, Kudo M (2018) REACH-2: a randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J Clin Oncol 36(15_suppl):4003–4003. https://doi.org/10.1200/JCO.2018.36.15_suppl.4003
Nicholson LJ, Smith PR, Hiller L, Szlosarek PW, Kimberley C, Sehouli J, Koensgen D, Mustea A, Schmid P, Crook T (2009) Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer 125(6):1454–1463. https://doi.org/10.1002/ijc.24546
Acknowledgements
We would like to thank Marinela Capanu for her critical review of the manuscript.
Funding
The trial was funded by Polaris Pharmaceuticals, Inc. This research was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xiaoxing Feng, Amanda Johnston, and John Bomalaski are employees of Polaris Pharmaceuticals Inc. There are no other conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Harding, J.J., Do, R.K., Dika, I.E. et al. A phase 1 study of ADI-PEG 20 and modified FOLFOX6 in patients with advanced hepatocellular carcinoma and other gastrointestinal malignancies. Cancer Chemother Pharmacol 82, 429–440 (2018). https://doi.org/10.1007/s00280-018-3635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3635-3