Abstract
Purpose
We report our institutional observations of ten patients with advanced hepatocellular carcinoma (HCC) (seven and three were Child–Pugh class A and B, respectively) who received compassionate regorafenib therapy between June 2016 and January 2017. These patients did not fit the rigid criteria of a clinical trial and represented the use of regorafenib in an everyday clinic situation.
Methods
Regorafenib (160 mg P.O. daily) was administered to patients on a 4-week cycle (3 weeks on, 1 week off) until disease progression (assessed using mRECIST criteria) or discontinuation secondary to toxicity (assessed using CTCAE criteria). Relevant clinical data were abstracted from patient medical records and reviewed retrospectively.
Results
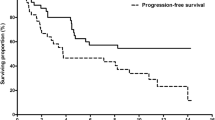
The median duration of patient treatment was 6.6 weeks, and the median time to disease progression was 12.5 weeks. Most common treatment emergent adverse events were fatigue, diarrhea, and hand–foot skin reaction. Elevated AST and ALT were the most commonly observed laboratory-assessed adverse events, which reached grade 3 status in the Child–Pugh class B patients only. We observed intolerance to regorafenib treatment in one patient who had previously received a liver transplant. We also saw lithium toxicity in one patient receiving long-term lithium treatment, suggesting a potential and unexpected drug–drug interaction with regorafenib.
Conclusions
Taken together, our observations indicate that regorafenib is beneficial in the treatment of patients with advanced HCC who progressed on or demonstrated intolerance to sorafenib therapy; however, careful selection and close monitoring of patients is necessary to maximize the benefit while minimizing the toxicities of regorafenib treatment.

Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal JA (2015) Global cancer statistics, 2012. CA: A Cancer J Clin 65:87–108. doi:10.3322/caac.21262
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362(9399):1907–1917. doi:10.1016/S0140-6736(03)14964-1
Llovet JM, Ricci S, Mazzaferro V, SHARP Investigators Study Group et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. doi:10.1056/NEJMoa0708857
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
Cheng AL, Kang YK, Lin DY et al (2013) Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 31(32):4067–4075. doi:10.1200/JCO.2012.45.8372
Johnson PJ, Qin S, Park JW et al (2013) Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 31(28):3517–3524. doi:10.1200/JCO.2012.48.4410
Cainap C, Qin S, Huang WT et al (2015) Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol 33(2):172–179. doi:10.1200/JCO.2013.54.3298
Zhu AX, Rosmorduc O, Evans TR et al (2015) SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 33(6):559–566. doi:10.1200/JCO.2013.53.7746
Abou-Alfa GK, Niedzwieski D, Knox JJ et al (2016) Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol 34(4S):192–192
Llovet JM, Decaens T, Raoul JL et al (2013) Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 31:3509–3516. doi:10.1200/JCO.2012.47.3009
Zhu AX, Kudo M, Assenat E et al (2014) Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 312:57–67. doi:10.1001/jama.2014.7189
Zhu AX, Park JO, Ryoo BY et al (2015) Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomized, double-blind, multicentre, phase 3 trial. Lancet Oncol 16:859–870. doi:10.1016/S1470-2045(15)00050-9
Bruix J, Qin S, Merle P et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
Wilhelm SM, Dumas J, Adnane L et al (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer;129(1):245–255. doi:10.1002/ijc.25864
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60. doi:10.1055/s-0030-1247132
Bruix J, Tak WY, Gasbarrini A et al (2013) Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer 49(16):3412–3419. doi:10.1016/j.ejca.2013.05.028
Drug safety labeling changes. US food and drug administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/203085s007lbl.pdf. Accessed on 23 May 2017
Tabchi S, Ghosn M (2015) Regorafenib: start low and go slow. Target Oncol 10(3):445–447
de’ Angelis N, Landi F, Nencioni M et al (2016) Role of sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation. Prog Transplant 26(4):348–355. doi:10.1177/1526924816664083
Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M (2012) High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int 25(11):1158–1164. doi:10.1111/j.1432-2277.2012.01540.x
Ketter TA, Frye MA, Cora-Locatelli G, Kimbrell TA, Post RM (1999) Metabolism and excretion of mood stabilizers and new anticonvulsants. Cell Mol Neurobiol 19(4):511–514
Finn RS (2017) Outcomes with sorafenib (SOR) followed by regorafenib (REG) or placebo (PBO) for hepatocellular carcinoma (HCC): Results of the international, randomized phase 3 RESORCE trial. J Clin Oncol 35(4S):344–344. doi:10.1200/JCO.2017.35.4_suppl.344
Belum VR, Wu S, Lacouture ME (2013) Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs 31(4):1078–1086. doi:10.1007/s10637-013-9977-0
Grothey A, Sobrero AF, Siena S et al (2013) Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol 31:3637–3637. doi:10.1200/jco.2013.31.15_suppl.3637
Mross K, Frost A, Steinbild S et al (2012) A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 18(9):2658–2667. doi:10.1158/1078-0432.CCR-11-1900
Ducreux M, Öhler L, Scheithauer W et al (2017) Safety and effectiveness of regorafenib (REG) in patients with metastatic colorectal cancer (mCRC) in routine clinical practice: an interim analysis (IA) from the prospective, observational CORRELATE study. J Clin Oncol 35(4S):700–700. doi:10.1200/JCO.2017.35.4_suppl.700
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Aiwu Ruth He serves as a speaker in Bayer’s speaker’s program. All other authors have no conflicts of interest to report. There is no disclosure of potential conflicts of interest among all authors.
Research involving human participants and/or animals
The study involves medical chart review of patients. The study is carried out under IRB-approved protocol with the titile of “Establishment of the High Quality Tumor Biobank and Clinical Database (2007- 345)”.
Informed consent
Informed consent is waived for this retrospective medical chart review.
Funding
No funding is obtained for this study.
Rights and permissions
About this article
Cite this article
Kim, K., Jha, R., Prins, P.A. et al. Regorafenib in advanced hepatocellular carcinoma (HCC): considerations for treatment. Cancer Chemother Pharmacol 80, 945–954 (2017). https://doi.org/10.1007/s00280-017-3431-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3431-5