Abstract
Purpose
To confirm the efficacy and safety of cetuximab and docetaxel in postoperative radiotherapy for high-risk head and neck cancer patients who cannot to be administered high-dose cisplatin.
Patients and methods
The eligibility criteria required stage III–IVB head and neck cancer patients who had undergone total resection, and for whom pathological evaluation revealed positive or close margins in the primary site and/or extracapsular nodal extension and/or two or more nodal metastases. In each case, the patients general condition prevented the use of high-dose cisplatin. Instead, they received cetuximab and docetaxel every week during a 66.6 Gy course of postoperative radiotherapy.
Results
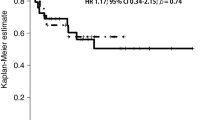
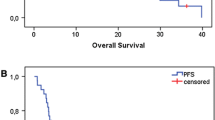
Eleven patients were enrolled; the median follow-up period was 22 months, and the 1- and 2-year disease free survival rates were 91 and 55%, respectively. Grade 3 adverse events included oral mucositis, radiation dermatitis, reduced white blood cell and neutrophil counts, lung infection, aspiration, and hyponatremia; however, no grade 4 adverse events were observed.
Conclusion
Administration of cetuximab and docetaxel during postoperative radiotherapy for high-risk poor condition head and neck cancer patients in poor general condition was both feasible and tolerable. With the safety of this treatment confirmed, we propose a phase trail to further clarify the efficacy of cetuximab and docetaxel use for high-risk cisplatin-intolerant patients.

Similar content being viewed by others
References
Cooper JS, Pajak TF, Forastiere AA et al (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350:1937–1944
Bernier J, Domange C, Ozsahin M et al (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350:1945–1952
Bernier J, Cooper JS, Pajak TF et al (2005) Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG #9501). Head Neck 27:843–850
Harari PM, Harris J, Kies MS et al (2014) Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: radiation therapy oncology group RTOG-0234. J Clin Oncol 32:2486–2495
Nishimura G, Tsukuda M, Horiuchi C et al (2007) Decrease of creatinine clearance rate with ageing in patients with head and neck cancer in Japan. Int J Clin Oncol 12:120–124
Hori M, Matsuda T, Shibata A et al (2015) Cancer incidence and incidence rate in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 45:884–891
Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. http://ganjoho.jp/reg_stat/
Tupchong L, Scott CB, Blitzer PH et al (1991) Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: long-term follow-up of RTOG study 73-03. Int J Radiat Oncol Biol Phys 20:21–28
Peters LJ, Goepfert H, Ang KK et al (1993) Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys 26:3–11
Bonner JA, Harari PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Bonner JA, Harari PM, Giralt J et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11:21–28
Vermorken JB, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Nakata E, Hunter N, Mason K et al (2004) C225 antiepidermal growth factor receptor antibody enhances the efficacy of docetaxel chemotherapy. Int J Radiat Oncol Biol Phys 59:1163–1173
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nishimura, G., Shiono, O., Sano, D. et al. Efficacy and safety of postoperative bio-chemoradiotherapy using cetuximab and docetaxel for high-risk head and neck cancer patients in Japan. Cancer Chemother Pharmacol 80, 203–207 (2017). https://doi.org/10.1007/s00280-017-3352-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3352-3