Abstract
Purpose
To assess the efficacy and toxicity of S-1 and bevacizumab combination therapy for patients previously treated for advanced non-squamous non-small cell lung cancer (NSCLC).
Methods
This was a prospective, multi-center, single-arm phase II study. Patients with non-squamous NSCLC who had experienced progression after cytotoxic chemotherapy were enrolled. Oral S-1 was administered on days 1–14 of a 21-day cycle, and bevacizumab (15 mg/kg) was given intravenously on day 1. Patients received S-1 adjusted on the basis of their creatinine clearance and body surface area. The primary endpoint was response rate (RR); secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety.
Results
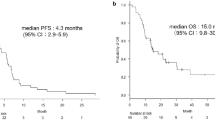
We enrolled 30 patients. One patient had never received platinum-based therapy. Five patients had activating mutations of the epidermal growth factor receptor gene, of whom four had received tyrosine kinase inhibitors before this study. The RR was 6.7% [95% confidence interval (CI) 1.8–21.3%], and the disease control rate (DCR) was 80% (95% CI 62.7–90.5%). Median PFS was 4.8 months (95% CI 2.7–6.4 months], and median OS was 13.8 months (95% CI 8.4 months–not applicable). Patients did not experience any Grade 4 toxicity or treatment-related death. Grade 3 hematologic toxicity (anemia) occurred in one patient (3.3%). The main Grade 3 non-hematologic toxicities were anorexia (10%), infection (10%), and diarrhea (6.7%).
Conclusion
The addition of bevacizumab to S-1 was tolerable, but not beneficial for patients with previously treated non-squamous NSCLC. We do not recommend further study of this regimen.



Similar content being viewed by others
References
Siegel R, Ma JM, Zou ZH, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S, Grp EGW (2014) Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:27–39
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18(10):2095–2103
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TCY, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22(9):1589–1597
Shiroyama T, Kijima T, Komuta K, Yamamoto S, Minami S, Ogata Y, Okafuji K, Imamura F, Hirashima T, Tachibana I, Kawase I, Kumanogoh A (2012) Phase II tailored S-1 regimen study of first-line chemotherapy in elderly patients with advanced and recurrent non-small cell lung cancer. Cancer Chemother Pharmacol 70(6):783–789
Totani Y, Saito Y, Hayashi M, Tada T, Kohashi Y, Mieno Y, Kato A, Imizu H, Yoneda Y, Hoshino T, Uchiyama Y, Takeuchi Y, Okazawa M, Sakakibara H (2009) A phase II study of S-1 monotherapy as second-line treatment for advanced non-small cell lung cancer. Cancer Chemother Pharmacol 64(6):1181–1185
Wada M, Yamamoto M, Ryuge S, Nagashima Y, Hayashi N, Maki S, Otani S, Katono K, Takakura A, Yanaihara T, Igawa S, Yokoba M, Mitsufuji H, Kubota M, Katagiri M, Masuda N (2012) Phase II study of S-1 monotherapy in patients with previously treated, advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 69(4):1005–1011
Shiroyama T, Komuta K, Imamura F, Hirashima T, Kijima T, Tachibana I, Kawase I (2011) Phase II study of S-1 monotherapy in platinum-refractory, advanced non-small cell lung cancer. Lung Cancer 74(1):85–88
Govindan R, Morgensztern D, Kommor MD, Herbst RS, Schaefer P, Gandhi J, Saito K, Zergebel C, Schiller J (2011) Phase II trial of S-1 as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 6(4):790–795
Sandler A, Yi J, Dahlberg S, Kolb MM, Wang LS, Hambleton J, Schiller J, Johnson DH (2010) Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 5(9):1416–1423
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M (2008) Safety evaluation of oral fluoropyrimidine S-1 for short- and long-term delivery in advanced gastric cancer: analysis of 3,758 patients. Cancer Chemother Pharmacol 61(2):335–343
Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, Takeda K, Inoue A, Tomii K, Harada M, Masuda N, Jiang H, Itoh Y, Ichinose Y, Saijo N, Fukuoka M (2008) Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26(26):4244–4252
Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A (2007) Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non-small-cell lung cancer. J Clin Oncol 25(30):4743–4750
Nishino K, Imamura F, Kumagai T, Katakami N, Hata A, Okuda C, Urata Y, Hattori Y, Tachihara M, Yokota S, Nishimura T, Kaneda T, Satouchi M, Morita S, Negoro S (2015) A randomized phase II study of bevacizumab in combination with docetaxel or S-1 in patients with non-squamous non-small-cell lung cancer previously treated with platinum based chemotherapy (HANSHIN Oncology Group 0110). Lung Cancer 89(2):146–153
Tokito T, Yamada K, Ichiki M, Takahashi K, Hisamatsu Y, Azuma K, Ishii H, Shukuya T, Takeoka H, Nishikawa K, Hoshino T (2015) A multicenter phase II study of S-1 combined with bevacizumab after platinum-based chemotherapy in patients with advanced non-squamous non-small cell lung cancer. J Clin Oncol 33(15):1
Nishino MM, Mok TSK, Nakagawa K, Yamamoto N, Shi Y-K, Zhang L, Lu S, Soo R, Yang J, Morita S, Sugawara S, Nokihara H, Takahashi T, Goto T, Chang J, Maemoto M, Ichinose Y, Cheng Y, Lim W-T, Tamura T (2016) EAST-LC: randomized controlled phase III trial of S-1 versus docetaxel in patients with non-small-cell lung cancer who had received a platinum-based treatment. Ann Oncol 27(Suppl_6):1218PD
Acknowledgements
The authors would like to thank Suguru Yamamoto, Moto Yaga, Kentaro Masuhiro, and Yumi Mitsuyama (Osaka Police Hospital), and Hiroshi Kida, Tomoyuki Otsuka, Osamu Morimura, Yoshiko Takeuchi, Akio Osa, and Mikako Ishijima (Osaka University Hospital) for their roles in patient enrollment and treatment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this work.
Conflict of interest
Tomonori Hirashima and his institution received research grants from Taiho Pharmaceutical Co., Ltd, and Chugai Pharmaceutical Co., Ltd. The other authors have declared no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nishijima-Futami, Y., Minami, S., Futami, S. et al. Phase II study of S-1 plus bevacizumab combination therapy for patients previously treated for non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol 79, 1215–1220 (2017). https://doi.org/10.1007/s00280-017-3321-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3321-x