Abstract
Purpose
Weekly dose-dense paclitaxel (PTX) in combination with carboplatin (CBDCA) every 3 weeks (ddTC therapy) is a standard treatment for patients with advanced ovarian cancer. However, there is no detailed analysis of the feasibility of ddTC therapy in elderly patients with ovarian cancer.
Methods
We identified patients diagnosed with ovarian, fallopian tube, or peritoneal cancer who received ddTC therapy at the National Cancer Center Hospital from April 2003 to April 2013. We assessed the feasibility of ddTC therapy in elderly patients aged 70 years or older (elderly group), comparing relative dose intensity (RDI) for PTX, CBDCA, and ddTC; adverse events; and rate of chemotherapy discontinuation to those in patients below 70 years of age (younger group).
Results
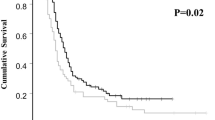
A total of 143 patients (elderly group, 22; younger group, 121) was analyzed. A comparison of RDI between these two groups showed no significant differences for PTX, CBDCA, and ddTC. Nonhematological and hematological toxicity profiles of the elderly and younger groups were similar, except that severe peripheral neuropathy (Grade 2 or higher) was more common in the elderly group. There was no significant difference in the rate of chemotherapy discontinuation (elderly group, 13.6 % vs. younger group, 7.4 %, p = 0.397).
Conclusions
Our study showed that ddTC therapy was feasible for elderly patients. However, to prevent severe neuropathy, PTX dose reduction deserves consideration.


Similar content being viewed by others
References
Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G (2007) Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol Off J Am Soc Clin Oncol 25(14):1858–1869. doi:10.1200/JCO.2006.10.4208
Efstathiou E, Dimopoulos MA, Bozas G, Kastritis E, Moulopoulos LA, Rodolakis A, Vlahos G, Gika D, Papadimitriou C, Bamias A (2007) Advanced epithelial ovarian cancer in the elderly: chemotherapy tolerance and outcome. Anticancer Res 27(1b):611–617
Fairfield KM, Murray K, Lucas FL, Wierman HR, Earle CC, Trimble EL, Small L, Warren JL (2011) Completion of adjuvant chemotherapy and use of health services for older women with epithelial ovarian cancer. J Clin Oncol Off J Am Soc Clin Oncol 29(29):3921–3926. doi:10.1200/JCO.2010.34.1552
Tew WP, Fleming GF (2015) Treatment of ovarian cancer in the older woman. Gynecol Oncol 136(1):136–142. doi:10.1016/j.ygyno.2014.10.028
Tew WP, Lichtman SM (2008) Ovarian cancer in older women. Semin Oncol 35(6):582–589. doi:10.1053/j.seminoncol.2008.08.007
Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374(9698):1331–1338. doi:10.1016/s0140-6736(09)61157-0
Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S, Terauchi F, Sugiyama T, Ochiai K (2013) Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14(10):1020–1026. doi:10.1016/s1470-2045(13)70363-2
Isonishi S, Yasuda M, Takahashi F, Katsumata N, Kimura E, Aoki D, Jobo T, Terauchi F, Tsuda H, Sugiyama T (2008) Randomized phase III trial of conventional paclitaxel and carboplatin (c-TC) versus dose dense weekly paclitaxel and carboplatin (dd-TC) in women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: Japanese Gynecologic Oncology. In: ASCO Annual Meeting; Chicago, IL, USA, 30 May–3 June 2008. Abstract 5506
Falandry C, Weber B, Savoye AM, Tinquaut F, Tredan O, Sevin E, Stefani L, Savinelli F, Atlassi M, Salvat J, Pujade-Lauraine E, Freyer G (2013) Development of a geriatric vulnerability score in elderly patients with advanced ovarian cancer treated with first-line carboplatin: a GINECO prospective trial. Ann Oncol Off J Eur Soc Med Oncol ESMO 24(11):2808–2813. doi:10.1093/annonc/mdt360
Pignata S, Breda E, Scambia G, Pisano C, Zagonel V, Lorusso D, Greggi S, De VR, Ferrandina G, Gallo C, Perrone F (2008) A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A Multicentre Italian Trial in Ovarian cancer (MITO-5) study. Crit Rev Oncol Hematol 66(3):229–236. doi:10.1016/j.critrevonc.2007.12.005
Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, Bologna A, Weber B, Raspagliesi F, Panici PB, Cormio G, Sorio R, Cavazzini MG, Ferrandina G, Breda E, Murgia V, Sacco C, Cinieri S, Salutari V, Ricci C, Pisano C, Greggi S, Lauria R, Lorusso D, Marchetti C, Selvaggi L, Signoriello S, Piccirillo MC, Di Maio M, Perrone F (2014) Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 15(4):396–405. doi:10.1016/s1470-2045(14)70049-x
(2004) Japanese translation of common terminology criteria for adverse events (CTCAE), and instructions and guidelines. Int J Clin Oncol 9(Suppl 3):1–82. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transp 48(3):452–458. doi:10.1038/bmt.2012.244
Akerley W, Herndon JE, Egorin MJ, Lyss AP, Kindler HL, Savarese DM, Sherman CA, Rosen DM, Hollis D, Ratain MJ, Green MR (2003) Weekly, high-dose paclitaxel in advanced lung carcinoma: a phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer 97(10):2480–2486. doi:10.1002/cncr.11375
Lichtman SM, Hurria A, Cirrincione CT, Seidman AD, Winer E, Hudis C, Cohen HJ, Muss HB (2012) Paclitaxel efficacy and toxicity in older women with metastatic breast cancer: combined analysis of CALGB 9342 and 9840. Ann Oncol Off J Eur Soc Med Oncol ESMO 23(3):632–638. doi:10.1093/annonc/mdr297
Tanabe Y, Hashimoto K, Shimizu C, Hirakawa A, Harano K, Yunokawa M, Yonemori K, Katsumata N, Tamura K, Ando M, Kinoshita T, Fujiwara Y (2013) Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int J Clin Oncol 18(1):132–138. doi:10.1007/s10147-011-0352-x
Chase DM, Huang H, Foss CD, Wenzel LB, Monk BJ, Burger RA (2015) Neurotoxicity in ovarian cancer patients on Gynecologic Oncology Group (GOG) protocol 218: characteristics associated with toxicity and the effect of substitution with docetaxel: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 136(2):323–327. doi:10.1016/j.ygyno.2014.12.021
Lee JJ, Swain SM (2006) Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol Off J Am Soc Clin Oncol 24(10):1633–1642. doi:10.1200/jco.2005.04.0543
Errico A (2014) Gynaecological cancer: MITO-7-a weekly regimen associated with a better quality of life for patients with advanced ovarian cancer. Nat Rev Clin Oncol 11(4):177. doi:10.1038/nrclinonc.2014.45
Kanesvaran R, Li H, Koo KN, Poon D (2011) Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol Off J Am Soc Clin Oncol 29(27):3620–3627. doi:10.1200/jco.2010.32.0796
Joseph N, Clark RM, Dizon DS, Lee MS, Goodman A, Boruta D Jr, Schorge JO, Del Carmen MG, Growdon WB (2015) Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol Oncol. doi:10.1016/j.ygyno.2015.03.052
Maas HA, Kruitwagen RF, Lemmens VE, Goey SH, Janssen-Heijnen ML (2005) The influence of age and co-morbidity on treatment and prognosis of ovarian cancer: a population-based study. Gynecol Oncol 97(1):104–109. doi:10.1016/j.ygyno.2004.12.026
Freyer G, Geay JF, Touzet S, Provencal J, Weber B, Jacquin JP, Ganem G, Tubiana-Mathieu N, Gisserot O, Pujade-Lauraine E (2005) Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol Off J Eur Soc Med Oncol ESMO 16(11):1795–1800. doi:10.1093/annonc/mdi368
Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP (2011) Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol Off J Am Soc Clin Oncol 29(25):3457–3465. doi:10.1200/JCO.2011.34.7625
Tredan O, Geay JF, Touzet S, Delva R, Weber B, Cretin J, Provencal J, Martin J, Stefani L, Pujade-Lauraine E, Freyer G, Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens (2007) Carboplatin/cyclophosphamide or carboplatin/paclitaxel in elderly patients with advanced ovarian cancer? Analysis of two consecutive trials from the Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. Ann Oncol Off J Eur Soc Med Oncol ESMO 18(2):256–262. doi:10.1093/annonc/mdl400
Acknowledgments
This research was partially supported by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED, No.: 15ck0106075h0002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bun, S., Yunokawa, M., Ebata, T. et al. Feasibility of dose-dense paclitaxel/carboplatin therapy in elderly patients with ovarian, fallopian tube, or peritoneal cancer. Cancer Chemother Pharmacol 78, 745–752 (2016). https://doi.org/10.1007/s00280-016-3100-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3100-0