Abstract
Background
The approved dose of gefitinib is fixed, without adjustment for physical size. We demonstrated previously that its efficacy was affected by body surface area (BSA) in patients with EGFR-mutant non-small cell lung cancer (NSCLC). To validate these observations, we assessed the association between BSA and the efficacy of gefitinib using a different patient cohort.
Methods
Prospective cohort data from 115 NSCLC patients with EGFR-mutant tumours, who received gefitinib monotherapy between 2007 and 2012, were analysed.
Results
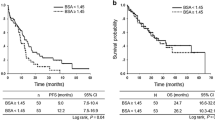
Gefitinib was less effective in individuals with a high BSA (≥1.5 m2) in EGFR-mutant NSCLC compared with those with a low BSA (<1.5 m2). The median progression-free survival (PFS) in the high- and low-BSA groups was 4.2 and 8.5 months, respectively, although there was no difference in survival among the whole NSCLC cohort. Multivariate analysis also showed a significant effect of BSA on PFS (hazard ratio 1.72; 95 % confidence interval 1.08–2.74; p = 0.021). Sensitivity analysis revealed that the use of the BSA cut-off level around 1.50 m2 was robust for detecting subpopulations that would benefit less from gefitinib monotherapy.
Conclusion
We found in the prospective cohort data that BSA could affect the efficacy of gefitinib monotherapy in patients with EGFR-mutant NSCLC, suggesting that BSA-based dose setting of gefitinib monotherapy might be further investigated, despite the fact that no molecular-targeted agent described to date undergoes dose adjustment according to BSA.

Similar content being viewed by others
References
Lynch TJ, BellDW Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Pao W, Miller V, Zakowski M et al (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101:13306–13311
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Mitsudomi T, Morita S, Yatabe Y, et al for West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Maemondo M, Inoue A, Kobayashi K et al; for North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Fukuoka M, Yano S, Giaccone G et al (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol 21:2237–2246
Kris MG, Natale RB, Herbst RS et al (2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290:2149–2158
Ichihara E, Hotta K, Hisamoto A et al (2012) Impact of body surface area on efficacy of gefitinib in patients with non-small cell lung cancer harboring activating epidermal growth factor receptor mutation. J Clin Oncol 30(suppl; abstr 2607)
DuBois D, DuBois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Ichihara E, Hotta K, Takigawa N et al (2013) Impact of physical size on gefitinib efficacy in patients with non-small cell lung cancer harboring EGFR mutations. Lung Cancer 81:435–439
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Igawa S, Kasajima M, Ishihara M et al (2014) Evaluation of gefitinib efficacy according to body surface area in patients with non-small cell lung cancer harboring an EGFR mutation. Cancer Chemother Pharmacol 74:939–946
Larson RA, Druker BJ, Guilhot F et al (2008) Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 111:4022–4028
Kawaguchi T, Hamada A, Hirayama C et al (2009) Relationship between an effective dose of imatinib, body surface area, and trough drug levels in patients with chronic myeloid leukemia. Int J Hematol 89:642–648
Nakamura Y, Sano K, Soda H et al (2010) Pharmacokinetics of gefitinib predicts antitumor activity for advanced non-small cell lung cancer. J Thorac Oncol 5:1404–1409
Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P (2010) The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One 5:e8933
Acknowledgments
The interpretation and reporting of these data are the sole responsibility of the authors. K.K. and K.H. had full access to all of the data in the study, were responsible for the integrity of the data and accuracy of the data analysis and contributed to the study design, data collection, analyses and manuscript writing. All other co-authors contributed to the manuscript writing. The authors would like thank Dr. Kiichiro Ninomiya for his helpful comments in the analysis of the results.
Conflict of interest
K.H. has received honoraria from Pfizer, Eli Lilly Japan, Sanofi, Daiichi-Sankyo Pharmaceutical and Chugai Pharmaceutical. NT received honoraria from AstraZeneca, Chugai Pharmaceutical Company and Boehringer-Ingelheim in Japan. KK received honoraria from Eli Lilly Japan, Nihon Kayaku, AstraZeneca, Daiichi-Sankyo Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical and Sanofi-Aventis. All other authors declared no conflicts of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kudo, K., Hotta, K., Ichihara, E. et al. Impact of body surface area on survival in EGFR-mutant non-small cell lung cancer patients treated with gefitinib monotherapy: observational study of the Okayama Lung Cancer Study Group 0703. Cancer Chemother Pharmacol 76, 251–256 (2015). https://doi.org/10.1007/s00280-015-2789-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2789-5