Abstract
Background
The population of elderly patients with lung cancer is increasing worldwide. Although first-line gefitinib is one of the standard treatments for advanced non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutation, few data have been reported regarding gefitinib and elderly patients.
Patients and methods
Chemotherapy-naïve patients aged 70 years or older with stage IIIB or IV NSCLC harboring EGFR-activating mutation were enrolled and treated with 250 mg of gefitinib daily until disease progression. The primary end point was response rate, and secondary end points were survival, safety, and quality of life.
Results
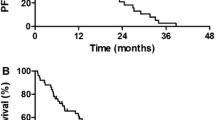
Twenty patients were enrolled, and the median age was 79.5 years (range 72–90). Overall response rate was 70 % (95 % CI 45.7–88.1 %), and the disease control rate was 90 % (95 % CI 68.3–98.7 %). The median progression-free survival and overall survival time were 10.0 and 26.4 months, respectively. The Functional Assessment of Cancer Therapy-Lung Cancer Subscale (FACT-LCS) scores improved significantly 4 weeks after the initiation of gefitinib (P = 0.037) and maintained favorably over a 12-week assessment period. Among the seven items of FACT-LCS, shortness of breath and cough improved significantly after 4 weeks of treatment (P = 0.046 and P = 0.008, respectively). The most common adverse events were rash and liver dysfunction. Although Grade 1 pneumonitis developed in one patient, no treatment-related death was observed.
Conclusion
First-line gefitinib therapy is effective and feasible for elderly patients harboring EGFR mutation, and improves disease-related symptoms, especially pulmonary symptoms like shortness of breath and cough.



Similar content being viewed by others
References
Quoix E, Westeel V, Zalcman G, Milleron B (2011) Chemotherapy in elderly patients with advanced non-small cell lung cancer. Lung Cancer 74:364–368
Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2013) SEER cancer statistics review, 1975–2010. National Cancer Institute. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013
Pallis AG, Karampeazis A, Vamvakas L, Vardakis N, Kotsakis A, Bozionelou V, Kalykaki A, Hatzidaki D, Mavroudis D, Georgoulias V (2011) Efficacy and treatment tolerance in older patients with NSCLC: a meta-analysis of five phase III randomized trials conducted by the Hellenic Oncology Research Group. Ann Oncol 22:2448–2455
The Elderly Lung Cancer Vinorelbine Italian Study Group (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 91:66–72
Kudoh S, Takeda K, Nakagawa K, Takada M, Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, Seto T, Ando M, Satoh T, Yoshimura N, Negoro S, Fukuoka M (2006) Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 24:3657–3663
Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, Barbera S, Ferraù F, Piazza E, Rosetti F, Clerici M, Bertetto O, Robbiati SF, Frontini L, Sacco C, Castiglione F, Favaretto A, Novello S, Migliorino MR, Gasparini G, Galetta D, Iaffaioli RV, Gebbia V, MILES Investigators (2003) Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 95:362–372
Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L, Dansin E, Poudenx M, Molinier O, Vaylet F, Moro-Sibilot D, Herman D, Bennouna J, Tredaniel J, Ducoloné A, Lebitasy MP, Baudrin L, Laporte S, Milleron B, Intergroupe Francophone de Cancérologie Thoracique (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 378:1079–1088
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Morita S, Okamoto I, Kobayashi K, Yamazaki K, Asahina H, Inoue A, Hagiwara K, Sunaga N, Yanagitani N, Hida T, Yoshida K, Hirashima T, Yasumoto K, Sugio K, Mitsudomi T, Fukuoka M, Nukiwa T (2009) Combined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutations. Clin Cancer Res 15:4493–4498
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Maemondo M, Minegishi Y, Inoue A, Kobayashi K, Harada M, Okinaga S, Morikawa N, Oizumi S, Tanaka T, Isobe H, Kudoh S, Hagiwara K, Nukiwa T, Gemma A (2012) First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol 7:1417–1422
Fujita S, Katakami N, Masago K, Yoshioka H, Tomii K, Kaneda T, Hirabayashi M, Kunimasa K, Morizane T, Mio T (2012) Customized chemotherapy based on epidermal growth factor receptor mutation status for elderly patients with advanced non-small-cell lung cancer: a phase II trial. BMC Cancer 12:185
Asami K, Koizumi T, Hirai K, Ameshima S, Tsukadaira A, Morozumi N, Morikawa A, Atagi S, Kawahara M (2011) Gefitinib as first-line treatment in elderly epidermal growth factor receptor-mutated patients with advanced lung adenocarcinoma: results of a Nagano Lung Cancer Research Group study. Clin Lung Cancer 12:387–392
Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N, Duffield EL, Rukazenkov Y, Speake G, Jiang H, Armour AA, To KF, Yang JC, Mok TS (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29:2866–2874
Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, Wolf MK, Johnson DH (2002) What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol 55:285–295
Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, Liao M, Chen YM, Kuo HP, Negoro S, Lam KC, Armour A, Magill P, Fukuoka M (2011) Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol 6:1872–1880
Oizumi S, Kobayashi K, Inoue A, Maemondo M, Sugawara S, Yoshizawa H, Isobe H, Harada M, Kinoshita I, Okinaga S, Kato T, Harada T, Gemma A, Saijo Y, Yokomizo Y, Morita S, Hagiwara K, Nukiwa T (2012) Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oncologist 17:863–870
Cella D, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, Heyes A, Ochs JS, Wolf MK, Kay AC, Kris MG, Natale RB (2005) Clinically meaningful improvement in symptoms and quality of life for patients with non-small-cell lung cancer receiving gefitinib in a randomized controlled trial. J Clin Oncol 23:2946–2954
Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA (2008) Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 26:2350–2357
Ranson M, Hammond LA, Ferry D, Kris M, Tullo A, Murray PI, Miller V, Averbuch S, Ochs J, Morris C, Feyereislova A, Swaisland H, Rowinsky EK (2002) ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol 20:2240–2250
Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, Eckhardt SG, Tolcher A, Britten CD, Denis L, Ferrante K, Von Hoff DD, Silberman S, Rowinsky EK (2001) Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19:3267–3279
Takahashi K, Saito H, Hasegawa Y, Ogasawara T, Taniguchi H, Suzuki R, Yamamoto M, Shindoh J, Yatabe Y, Shimokata K (2009) A phase II study of gefitinib monotherapy as first-line treatment for elderly patients with stage IIIB/IV adenocarcinoma of the lung. Eur J Cancer Suppl 7:547
Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, Ariyoshi Y, Fukuoka M (2006) Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 24:2549–2556
Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, Tsuboi M, Yokota S, Nakagawa K, Suga M, Japan Thoracic Radiology Group, Jiang H, Itoh Y, Armour A, Watkins C, Higenbottam T, Nyberg F (2008) Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 177:1348–1357
Conflict of interest
Kosuke Takahashi, Hiroshi Saito, Yoshinori Hasegawa, Yasuteru Sugino, and Joe Shindoh received honoraria from AstraZeneca. Yoshinori Hasegawa received research funding for his institute from AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
This trial is registered at UMIN-CTR, Number UMIN000001863.
Rights and permissions
About this article
Cite this article
Takahashi, K., Saito, H., Hasegawa, Y. et al. First-line gefitinib therapy for elderly patients with non-small cell lung cancer harboring EGFR mutation: Central Japan Lung Study Group 0901. Cancer Chemother Pharmacol 74, 721–727 (2014). https://doi.org/10.1007/s00280-014-2548-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2548-z