Abstract
Purpose
PRO95780, a human monoclonal antibody (mAb) against death receptor 5 (DR5/TRAIL-R2/TNFRSF10B), was developed for the treatment for cancer. Our objective was to characterize pharmacokinetics (PK) in mice, rats, and cynomolgus monkeys and concentration–effect relationships of PRO95780 in xenograft mouse models of human cancers; this would guide the selection of dose and regimen for clinical trials.
Methods
The PK profiles were determined in mice, rats, and cynomolgus monkeys. Three xenograft models with a wide range of in vitro sensitivities to PRO95780 were selected for efficacy studies. Tumoristatic serum concentrations (TSCs) were determined using PK/pharmacodynamic (PD) modeling with tumor growth as a PD endpoint. A species-invariant time PK scaling method was employed to estimate disposition in humans using PK data in cynomolgus monkeys. Furthermore, the predicted human PK parameters were used to estimate dose and regimen to achieve TSC observed in mice at the steady-state trough concentrations (C trough ss) in the clinic.
Results
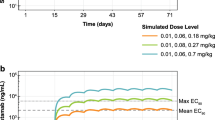
Linear PK was observed across species. A serum concentration of 22 μg/mL was identified to be the target TSC in mice. A dose of 10 mg/kg administered once every 2 weeks (Q2W) was predicted to achieve a TSC at C trough ss in 95 % of patients.
Conclusions
PRO95780 has linear PK in mice, rats, and monkeys. Estimated TSCs varied among different xenograft models. A projected target dose in humans is achievable for Q2W administration within the dose range used for other commercial mAbs.






Similar content being viewed by others
References
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271(22):12687–12690
Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11(2):255–260
Griffith TS, Lynch DH (1998) TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol 10(5):559–563
Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L (1997) Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7(6):821–830
Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A (1997) Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem 272(22):14029–14032
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277(5327):815–818
Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J (1997) TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 7(6):831–836
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gracy CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277(5327):818–821
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT (1997) TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 16(17):5386–5397
Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG (1997) The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7(6):813–820
Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA (1997) Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 186(7):1165–1170
Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR (1998) Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 273(23):14363–14367
Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Askenazi P (1997) A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol 7(12):1003–1006
Ashkenazi A, Holland P, Eckhardt SG (2008) Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol 26(21):3621–3630
Takeda S, Iwai A, Nakashima M, Fujikura D, Chiba S, Li HM, Uehara J, Kawaguchi S, Kaya M, Nagoya S, Wada T, Yuan J, Raytor S, Ashworth A, Reed JC, Yamashita T, Uede T, Miyazaki T (2007) LKB1 is crucial for TRAIL-mediated apoptosis induction in osteosarcoma. Anticancer Res 27(2):761–768
Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, Compaan D, Lowman H, Hymowitz S, Askenazi A (2008) Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ 15(4):751–761
Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, Lawrence D, Zheng Z, Koeppen H, Stern H, Schwall R, Ashkenazi A (2008) Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res 14(23):7733–7740
Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Sager J, Ashkenazi A, Bray G, Mendelson D (2010) A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res 16(4):1256–1263
Jusko WJ (1971) Pharmacodynamics of chemotherapeutic effects: dose-time-response relationships for phase-nonspecific agents. J Pharm Sci 60(6):892–895
Dedrick RL (1973) Animal scale-up. J Pharmacokinet Biopharm 1(5):435–461
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S (2011) Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? mAbs 3(1):61–66
Ling J, Zhou H, Jiao Q, Davis HM (2009) Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol 49(12):1382–1402
Mahmood I (2004) Interspecies scaling of protein drugs: prediction of clearance from animals to humans. J Pharm Sci 93(1):177–185
Sharma V, McNeill JH (2009) To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 157(6):907–921
Kamath AV, Lu D, Gupta P, Jin D, Xiang H, Wong A, Leddy C, Crocker L, Schaefer G, Sliwkowski MX, Daminco-Beyer LA (2012) Preclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical dose. Cancer Chemother Pharmacol 69(4):1063–1069
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49(10):633–659
Jumbe NL, Xin Y, Leipold DD, Crocker L, Dugger D, Mai E, Sliwkowski MX, Fielder PJ, Tibbits J (2010) Modeling the efficacy of trastuzumab-DM1, an antibody drug conjugate, in mice. J Pharmacokinet Pharmacodyna 37(3):221–242
Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 299(1):31–38
Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N, McKeever K, Sliwkowski MX (2006) Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother 55(6):717–727
Daniels DL, Weis WI (2005) Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol 12(4):364–371
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberley RP, Zhou T (2001) Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 7(8):954–960
Lippa MS, Strockbine LD, Le TT, Branstetter DG, Strathdee CA, Holland PM (2007) Expression of anti-apoptotic factors modulates Apo2L/TRAIL resistance in colon carcinoma cells. Apoptosis 12(8):1465–1478
Kang Z, Chen JJ, Yu Y, Li B, Sun SY, Zhang B, Cao L (2011) Drozitumab, a human antibody to death receptor 5, has potent antitumor activity against rhabdomyosarcoma with the expression of caspase-8 predictive of response. Clin Cancer Res 17(10):3181–3192
Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V, Diamond P, Zannettino AC, Findlay DM, Evdokiou A (2009) Apomab, a fully human agonistic antibody to DR5, exhibits potent antitumor activity against primary and metastatic breast cancer. Mol Cancer Ther 8(10):2969–2980
Acknowledgments
We thank Shannon Stainton, Mike Reich, Amy Oldendorp, and Michelle Gonzales from in vivo studies group in the Safety Assessment Department at Genentech Inc. for performing efficacy and PK studies and the BioAnalytical Assays Department at Genentech Inc. for providing PK assay data. The authors wish to thank Drs. Frank P. Theil, Saileta Prabhu, and Leslie A. Khawli and others from Genentech Inc. for reviewing the manuscript. The authors thank PRO95780 project team members for their valuable discussion.
Conflict of interest
Authors are employees at Genentech/Roche and hold stock in Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, H., Reyes, A.E., Eppler, S. et al. Death receptor 5 agonistic antibody PRO95780: preclinical pharmacokinetics and concentration–effect relationship support clinical dose and regimen selection. Cancer Chemother Pharmacol 72, 405–415 (2013). https://doi.org/10.1007/s00280-013-2200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2200-3