Abstract
Background
Combining anti-angiogenesis agents with cytotoxic agents for the treatment of malignant gliomas may affect the cytotoxic drug distribution by normalizing the blood–brain barrier (BBB). This study examines the intratumoral concentration of temozolomide (TMZ) in the presence and absence of the pan-VEGF receptor tyrosine kinase inhibitor, cediranib.
Methods
Seven nude rats bearing U87 intracerebral gliomas had a microdialysis probe centered within the tumor. Ten-days after tumor implantation, TMZ (50 mg/kg) was given orally. The extracellular fluid (ECF) concentrations of TMZ within the tumor were assessed via microdialysis for 6 h following TMZ administration. Cediranib (6 mg/kg) was then given orally, and 12 h later, TMZ was re-administered with subsequent microdialysis collection. A subset of animals also underwent functional MRI to assess angiogenesis in vivo at post-inoculation days 12 and 21, before and after the cediranib treatment.
Results
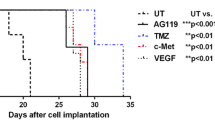
After dosing of oral TMZ only, ECF-TMZ mean-C max and area under the concentration curve(AUC0–∞) within the tumor were 0.59 μg/mL and 1.82 μg h/mL, respectively. Post-cediranib, ECF-TMZ mean-C max and AUC0–∞ were 0.83 μg/mL and 3.72 ± 0.61 μg h/mL within the tumor, respectively. This represented a 1.4-fold (p = 0.3) and 2.0-fold (p = 0.06) increase in the ECF-TMZ C max and AUC0–∞, respectively, after cediranib administration. In vivo MRI measurements of the various vascular parameters were consistent with a BBB “normalization” profile following cediranib treatment.
Conclusions
In the U87 intracerebral glioma model, within the first day of administration of cediranib, the intratumoral concentrations of TMZ in tumor ECF were slightly, but not statistically significantly, increased when compared to the treatment of TMZ alone with radiographic evidence of a normalized BBB.



Similar content being viewed by others
References
Ahluwalia MS (2011) 2010 Society for Neuro-Oncology Annual Meeting: a report of selected studies. Expert Rev Anticancer Ther 11:161–163
Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H (2008) Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol 15:2887–2893
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG, New Approaches to Brain Tumor Therapy Consortium (NABTT) (2009) Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol 91:51–58
Bungay PM, Morrison PF, Dedrick RL (1990) Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci 46:105–119
Farrar CT, Kamoun WS, Ley CD, Kim YR, Catana C, Kwon SJ, Rosen BR, Jain RK, Sorensen AG (2011) Sensitivity of MRI tumor biomarkers to VEGFR inhibitor therapy in an orthotopic mouse glioma model. PLoS ONE 6:e17228
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Gibaldi MPD (1982) Pharmacokinetics. Dekker, New York
Grossman R, Rudek MA, Brastianos H, Zadnik P, Brem H, Tyler B, Blakeley JO (2012) The impact of bevacizumab on temozolomide concentrations in intracranial U87 gliomas. Cancer Chemother Pharmacol 70:129–139
Jacobs S, McCully CL, Murphy RF, Bacher J, Balis FM, Fox E (2010) Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother Pharmacol 65:817–824
Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, Batchelor TT, di Tomaso E, Duda DG, Munn LL, Fukumura D, Sorensen AG, Jain RK (2009) Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol 27:2542–2552
Kim KJ, Wang L, Su YC, Gillespie GY, Salhotra A, Lal B, Laterra J (2006) Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res 12:1292–1298
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Le Quellec A, Dupin S, Genissel P, Saivin S, Marchand B, Houin G (1995) Microdialysis probes calibration: gradient and tissue dependent changes in no net flux and reverse dialysis methods. J Pharmacol Toxicol Methods 33:11–16
Ma J, Li S, Reed K, Guo P, Gallo JM (2003) Pharmacodynamic-mediated effects of the angiogenesis inhibitor SU5416 on the tumor disposition of temozolomide in subcutaneous and intracerebral glioma xenograft models. J Pharmacol Exp Ther 305:833–839
Ma J, Pulfer S, Li S, Chu J, Reed K, Gallo JM (2001) Pharmacodynamic-mediated reduction of temozolomide tumor concentrations by the angiogenesis inhibitor TNP-470. Cancer Res 61:5491–5498
McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quinones-Hinojosa A (2009) Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 110:583–588
Pathak AP (2009) Magnetic resonance susceptibility based perfusion imaging of tumors using iron oxide nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1:84–97
Pathak AP, Hochfeld WE, Goodman SL, Pepper MS (2008) Circulating and imaging markers for angiogenesis. Angiogenesis 11:321–335
Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW (2009) The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res 15:7092–7098
Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK (2009) A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69:5296–5300
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jurgensmeier JM, Ogilvie DJ (2005) AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 65:4389–4400
Zhou Q, Gallo JM (2005) In vivo microdialysis for PK and PD studies of anticancer drugs. AAPS J 7:E659–E667
Acknowledgments
We would like to thank John Laterra, M.D., Bachchu Lal, Ph.D., Ming Zhao, Ph.D., Ping He, and Teresia Wanjiku, MHS, for their technical support. This work was supported by the American Physicians Fellowship (APF) for Medicine in Israel (Rachel Grossman), the American Cancer Society Research Scholar Grant (RSG-08-119-01-CCE), and by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 RR025005) and the Shared Instrument Grant (1S10RR026824-01).The work was also supported by the generosity of Peter and Ali Jennison. The project described was supported by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grossman, R., Tyler, B., Rudek, M.A. et al. Microdialysis measurement of intratumoral temozolomide concentration after cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, in a U87 glioma model. Cancer Chemother Pharmacol 72, 93–100 (2013). https://doi.org/10.1007/s00280-013-2172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2172-3