Abstract
Purpose
To characterize the hyperthermic intraperitoneal oxaliplatin (HIO) pharmacokinetics in peritoneum and plasma in patients with peritoneal carcinomatosis (PC) after cytoreductive surgery (CRS).
Methods
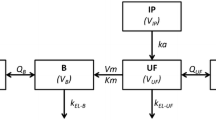
Data from 36 patients receiving HIO diluted in isotonic 4 % icodextrin were combined with data from 13 patients receiving HIO diluted in isotonic 5 % dextrose. Total oxaliplatin in peritoneal and plasma fluids were used to characterize an open two-compartment disposition model with linear distribution and elimination and first-order absorption from peritoneum to plasma using NONMEM software. The effect of patient- and treatment-related covariates on oxaliplatin pharmacokinetic parameters was explored.
Results
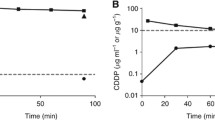
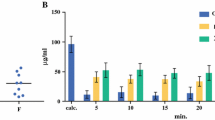
The typical value (interindividual variability, %) in k a , CL, and V ss were 0.57 h−1 (43 %), 1.71 L h−1 (39 %), and 77 L (65 %), respectively. No significant effect of age, body surface area, sex, creatinine clearance, liver metastases, PC index, and complete cytoreduction on pharmacokinetic parameters was found. A 12–15 % reduction in peritoneal volume of distribution was observed in patients receiving HIO diluted in 5 % dextrose relative to those patients receiving HIO diluted in 4 % icodextrin.
Conclusions
The integration of peritoneal and plasma data demonstrated oxaliplatin linear absorption from peritoneum to plasma, non-specific distribution to a peripheral compartment, and linear elimination from the central compartment when HIO was administered with isotonic carrier solutions to PC patients who underwent CRS. Only the effect of the carrier solution had an impact in the peritoneal volume of distribution, but its clinical relevance seems to be limited, especially for short HIO infusions (<60 min).



Similar content being viewed by others
References
Spiliotis JD (2010) Peritoneal carcinomatosis cytoreductive surgery and HIPEC: a ray of hope for cure. Hepatogastroenterology 57:1173–1177
Valenzuela B, Nalda-Molina R, Bretcha-Boix P et al (2011) Pharmacokinetic and pharmacodynamic analysis of hyperthermic intraperitoneal oxaliplatin-induced neutropenia in subjects with peritoneal carcinomatosis. AAPS J 13:72–82
Elias D, Lefevre JH, Chevalier J et al (2009) Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 27:681–685
Di Giorgio A, Naticchioni E, Biacchi D et al (2008) Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 113:315–325
Verwaal VJ, van Ruth S, de Bree E et al (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21:3737–3743
Yang XJ, Huang CQ, Suo T et al (2011) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 18:1575–1581
Cao C, Yan TD, Black D et al (2009) A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surf Oncol 16:2152–2165
Sugarbaker PH (1998) Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 14:254–261
de Bree E, Tsiftsis D (2007) Principles of perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. Recent Results Cancer Res 169:39–51
Atallah D, Marsaud V, Radanyi C et al (2004) Thermal enhancement of oxaliplatin-induced inhibition of cell proliferation and cell cycle progression in human carcinoma cell lines. Int J Hyperthermia 20:405–419
Elias D, El Otmany A, Bonnay M et al (2002) Heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 13:267–272
Culy CR, Clemett D, Wiseman LR (2000) Oxaliplatin. A review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies. Drugs 60:895–924
Elias D, Sideris L (2003) Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg Oncol Clin North Am 12:755–769
Massari C, Brienza S, Rotarski M et al (2000) Pharmacokinetics of oxaliplatin in patients with normal versus impaired renal function. Cancer Chemother Pharmacol 45:157–164
Ferron G, Dattez S, Gladieff L et al (2008) Pharmacokinetics of heated intraperitoneal oxaliplatin. Cancer Chemother Pharmacol 62:679–683
Mohamed F, Sugarbaker PH (2003) Carrier solutions for intraperitoneal chemotherapy. Surg Oncol Clin North Am 12:813–824
Bretcha-Boix P, Farré-Alegre J, Sureda M et al (2010) Cytoreductive surgery and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colonic origin: outcomes after 7 years’ experience of a new centre for peritoneal surface malignancies. Clin Transl Oncol 12:437–442
Sugarbaker PH (1998) Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Sem Surg Oncol 14:254–261
Sugarbaker PH (1995) Peritonectomy procedures. Ann Surg 221:29–42
Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–374
Giacchetti S, Perpoint B, Zidani R et al (2000) Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first line treatment of metastatic colorectal cancer. J Clin Oncol 18:136–147
Dominici C, Petrucci F, Caroli S et al (1989) Pharmacokinetic study of high-dose continuous infusion cisplatin in children with solid tumors. J Clin Oncol 7:100–107
Sooriyaarachchi M, Narendran A, Gailer J (2011) Comparative hydrolysis and plasma protein binding of cis-platin and carboplatin in human plasma in vitro. Metallomics 3:49–55
Alimonti A, Petrucci F, Dominici C, Caroli S (1987) Determination of Pt in biological samples by inductively coupled plasma atomic emission spectrometry (ICP-AES) with electrothermal vaporization (ETV). J Trace Elem Electrolytes Health Dis 1:79–83
Beal SL, Sheiner LB, Boeckman AJ (1989–2006) NONMEM users guides. ICON Development Solutions, Ellicott City
Marzo A, Monti NC, Vuksic D (1999) Experimental, extrapolated and truncated areas under the concentration-time curve in bioequivalence trials. Eur J Clin Pharmacol 55:627–631
Bland JM, Altman DG (1996) Transformations, means, and confidence intervals. BMJ 312:1079
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biophar 20:511–528
Manly BFJ (1997) Randomization, bootstrap and Monte Carlo methods in biology. Texts in Statistical Science. Chapman and Hall, London
Wählby U, Jonsson EN, Karlsson MO (2001) Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 28:231–252
Efron B, Tibshirani R (1993) An introduction to the bootstrap. Chapman and Hall, London
Brendel K, Comets E, Laffont C et al (2006) Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23:2036–2049
Post TM, Freijer JI, Ploeger BA et al (2008) Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn 35:185–202
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24:2187–2197
Brouwers EE, Huitema AD, Beijnen JH et al (2008) Long-term platinum retention after treatment with cisplatin and oxaliplatin. BMC Clin Pharmacol 17(8):7
Stewart JH 4th, Shen P, Russell G et al (2008) A phase I trial of oxaliplatin for intraperitoneal hyperthermic chemoperfusion for the treatment of peritoneal surface dissemination from colorectal and appendiceal cancers. Ann Surg Oncol 15:2137–2145
Elias D, El Otmany A, Bonnay M et al (2002) Human pharmacokinetic study of heated intraperitoneal oxaliplatin in increasingly hypotonic solutions after complete resection of peritoneal carcinomatosis. Oncology 63:346–352
Litterst CL, Torres LJ, Sikic BI (1980) Absorption of antineoplastic drugs following large volume administration to rats. Cancer Treat Rep 66:147–155
Kusamura S, Dominique E, Baratti D et al (2008) Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol 98:247–252
Pestieau SR, Schnake KJ, Stuart OA et al (2001) Impact of carrier solutions on pharmacokinetics of intraperitoneal chemotherapy. Cancer Chemother Pharmacol 47:269–276
Acknowledgments
The authors would like to thank the patients, medical, nursing, and laboratory staff of the Hospital San Jaime who participated in the present study. This work was supported by Consellería de Sanidad of Comunidad Valenciana, Spain. Grant GE-079/11.
Conflict of interest
The author(s) indicated no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer: The views expressed in this article are the personal views of the authors reflecting their scientific knowledge of this topic and should not be understood or quoted as being made on behalf of the companies where the authors currently work.
Rights and permissions
About this article
Cite this article
Pérez-Ruixo, C., Valenzuela, B., Peris, J.E. et al. Population pharmacokinetics of hyperthermic intraperitoneal oxaliplatin in patients with peritoneal carcinomatosis after cytoreductive surgery. Cancer Chemother Pharmacol 71, 693–704 (2013). https://doi.org/10.1007/s00280-012-2060-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-2060-2