Abstract
Purpose
Nedaplatin (NDP), a platinum derivative, has been developed to reduce nephrotoxicity and gastrointestinal toxicity of cisplatin. The pharmacokinetic profile of NDP is similar to that of carboplatin (CBDCA). The optimal dosing for CBDCA is determined by the area under the curve (AUC) using Calvert’s formula. However, the administration dose of nedaplatin (NDP) is determined based on the body surface area in clinical treatment. Ishibashi et al. reported a formula for predicting NDP clearance based on renal function like Calvert’s formula for CBDCA. We conducted the present study to evaluate the Ishibashi’s formula.
Methods
A total of 22 patients with cervical or ovarian cancer, who underwent chemotherapy consisting of NDP and irinotecan (CPT-11), were examined in this study. Blood samples were collected at 0, 1, 2, 4, and 6 h after the end of infusion of NDP (48–80 mg/m2), and free platinum concentrations were measured. Observed AUCs were compared with predicted AUCs, which were calculated by the Ishibashi’s formula. In addition, the relative reduction in platelets (PLTs) was assessed as a parameter of adverse effects.
Results
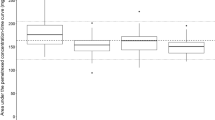
The observed AUC of NDP ranged from 4 to 14 (μg h−1 ml−1) with large variation. The predicted AUC based on renal function was correlated with the observed AUC. There was a relationship between observed AUC and the decrease in PLTs.
Conclusions
Ishibashi’s formula would be predictable and useful for estimating the individual dose of NDP.




Similar content being viewed by others
References
Niioka T, Uno T, Yasui-Furukori N, Takahata T, Shimizu M, Sugawara K, Tateishi T (2007) Pharmacokinetics of low-dose nedaplatin and validation of AUC prediction in patients with non-small-cell lung carcinoma. Cancer Chemother Pharmacol 59:575–580
Koenuma M, Kasai H, Uchida N, Takeda Y, Shiratori O, Muraoka Y, Totani T (1995) Antitumor activity of a new platinum complex, nedaplatin. Clin Rep 29:3213–3222
Weiss RB, Christian MC (1993) New cispaltin analogue in development. Drugs 46:360–377
Inuyama Y, Miyake H, Horiuchi M, Hayasaki K, Komiyama S, Ota K (1992) An early phase II clinical study of cis-diammine glycolato platinum, 254-s, for head and neck cancers. Jpn J Cancer Chemother 19:863–869
Inuyama Y, Hirosato M, Horiuchi M, Hayasaki K, Komiyama S, Ota K (1992) A late phase II clinical study of cis-diammine glycolato platinum, 254-s, for head and neck cancers. Jpn J Cancer Chemother 19:871–877
Furuse K, Fukuoka M, Kurita Y, Ariyoshi Y, Niitani H, Yoneda S, Fujii M, Hasegawa K, Nishiwaki Y, Tamura M, Kimura I, Inoue S, Oshima S, Kusume K, Sugimoto K (1992) A phase II clinical study of cis-diammine glycolato platinum, 254-S, for plimary lung cancer. Jpn J Cancer Chemother 19:879–884
Taguchi T, Wakui A, Nabeya K, Kurihara M, Isono K, Kakegawa T, Ota K (1992) A phase II clinical study of cis-diammine glycolato platinum, 254-S, for gastrointestinal cancers. Jpn J Cancer Chemother 19:483–488
Akaza H, Togashi M, Nishio Y, Miki T, Kotake T, Matsumura Y, Yoshida O, Aso Y (1992) Phase II study of cis-diammine (glycolato) platinum, 254-S, in patients with advanced germ-cell testicular cancer, prostatic cancer, and transitional-cell carcinoma of the urinary tract. 254-S urological cancer. Cancer Chemothe Pharmacol 31:187–192
Noda K, Ikeda M, Yakushiji M, Nishimura H, Terashima Y, Sasaki H, Hata T, Kuramoto H, Tanaka K, Takahashi T, Hirabayashi K, Yamabe T, Hatae M (1992) A phase II clinical study of cis-diammine glycolato platinum, 254-S, for cervical cancer of the uterus. Jpn J Cancer Chemother 19:885–892
Sugiyama T, Yakushiji M, Noda K, Ikeda M, Kudoh R, Yajima A, Tomoda Y, Terashima Y, Takeuchi S, Hiura M, Saji F, Takahashi T, Umesaki N, Sato S, Hatae M, Ohashi Y (2000) Phase II study of irinotecan and cisplatin as first-line chemotherapy in advanced or recurrent cervical cancer. Oncol 58:31–37
Machida S, Ohwada M, Fujiwara H, Konno R, Takano M, Kita T, Kikuchi Y, Komiyama S, Mikami M, Suzuki M (2003) Phase I study of combination chemothearpy using irinotecan hydrochloride and nedaplatin for advanced or recurrent cervical cancer. Oncology 65:102–107
Sugiyama T, Yakushiji M, Kamura T, Ikeda M, Umesaki N, Hasegawa K, Ishikawa M, Saji F, Hiura M, Takahashi T, Sato S, Oshiai K, Kikkawa F, Takeuchi S, Ohashi Y, Noda K, Japan CPT-11Study Group (2003) Irinotecan (CPT-11) and cisplatin as first line chemotherapy for advanced ovarian cancer. Oncology 63:16–22
Matsumura M, Takeshima N, Ota T, Omatsu K, Sakamoto K, Kawamata Y, Umayahara K, Tanaka H, Akiyama F, Takizawa K (2010) Neoadjuvant chemoteharpy followed by radical hysterectomy plus postoperative chemotherapy but no radiotherapy for stage IB2-IIB cervical cancer–Irinotecan and platinum chemotherapy. Gynecol Oncol 119:212–216
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756
Chatelut E, Canal P, Brunner V, Chevreau C, Boneu A, Roche H, Houin G, Bugat R (1995) Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 87:573–580
Ota K, Oguma T, Shimamura K (1994) Pharmacokinetics of platinum in cancer patients following intravenous infusion of cis-diammine (glycolate) platinum, 254-S. Anticancer Res 14:1383–1388
Sasaki Y, Tamura T, Taguchi K, Shinka T, Fujiwara Y, Fukuda M, Ohe Y, Bungo M, Horichi N, Niimi S, Minato K, Nakagawa K, Saijyo N (1989) Pharmacokinetics of (glycolato-O, O’)-diammine platinum(II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol 23:243–246
Ishibashi T, Yano Y, Oguma T (2002) A formula for predicting optimal dosage of nedaplatin based on renal function in adult cancer patients. Cancer Chemother Pharmacol 50:230–236
Ikeuchi I, Daikatsu K, Fujisawa I, Amano T (1990) Determination of platinum in biological materials by graphic furnace atomic absorption spectorometry. Iyakuhin Kenkyu 21:1082–1087
Ishibashi T, Yano Y, Oguma T (2003) Population pharmacokinetics of platinum after nedaplatin administration and model validation in adult patients. Br J Clin Pharmacol 56:205–213
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Duffull SB, Robinson BA (1997) Clinical pharmacokinetics and dose optimization of carboplatin. Clin Pharmacokinet 33:161–183
Ishibashi T, Yano Y, Oguma T (2005) Determination dosage for nedaplatin based on pharmacokinetic and toxycodynamic analysis. Anticancer Res 25:1273–1282
Conflict of interest
None of the authors has any former or present conflict of interest related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, S., Fujiwara, H., Oishi, T. et al. Evaluation of a formula for individual dosage of nedaplatin based on renal function. Cancer Chemother Pharmacol 69, 599–603 (2012). https://doi.org/10.1007/s00280-011-1739-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1739-0