Abstract
Purpose
The aim of this study was to determine the activity and toxicity of two sequential chemotherapy regimens in the first-line treatment of advanced non-small-cell lung cancer (NSCLC).
Methods
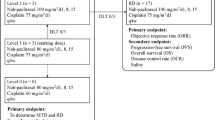
Eighty-eight chemonaive patients with stage IIIB/IV NSCLC were randomised to receive either three cycles of 75 mg/m2 cisplatin plus 75 mg/m2 docetaxel, both administered on day 1 every 21 days, followed by three cycles of 1,200 mg/m2 gemcitabine on days 1 and 8 every 3 weeks (arm A), or three cycles of 25 mg/m2 cisplatin plus 25 mg/m2 docetaxel on days 1, 8 and 15 every 28 days, followed by three cycles of 1,200 mg/m2 gemcitabine on days 1 and 8 every 3 weeks (arm B).
Results
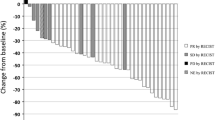
Of the evaluable patients, 61% in arm A (n = 41) and 36% (n = 44) in arm B completed treatment as per the protocol. The best tumour response rates were as follows (arm A and arm B): complete response: 2.4 and 2.3%; partial response: 39 and 20.4%; stable disease: 26.8 and 13.6%; and progressive disease: 31.8 and 45.4%. The median progression-free and overall survival were 3.9 and 12.3 months in arm A, respectively, 3.1 and 7.7 months in arm B. Grade 3–4 adverse events were more common in arm A. Grade 3–4 neutropenia was the main toxicity observed (56.1% in arm A and 11.4% in arm B).
Conclusions
Our data demonstrate the feasibility of a sequential approach of cisplatin plus docetaxel followed by single-agent gemcitabine. Weekly administration of platinum-docetaxel is associated with an improved safety profile but lower efficacy than the conventional three-weekly schedule (registration ID 2004-001044-72).



Similar content being viewed by others
References
Smith IE, O’Brien ME, Talbot DC et al (2001) Duration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol 19(5):1336–1343
Socinski MA, Schell MJ, Peterman A et al (2002) Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol 20(5):1335–1343
von Plessen C, Bergman B, Andresen O et al (2006) Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer 95(8):966–973
Park JO, Kim SW, Ahn JS et al (2007) Phase III trial of two versus four additional cycles in patients who are non progressive after two cycles of platinum based chemotherapy in non small-cell lung cancer. J Clin Oncol 25(33):5233–5239
Azzoli CG, Baker S Jr, Temin S et al (2009) American society of clinical oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 27(36):6251–6266
Soon YY, Stockler MR, Askie LM et al (2009) Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol 27(20):3277–3283
Ciuleanu T, Brodowicz T, Zielinski C et al (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374(9699):1432–1440
Cappuzzo F, Ciuleanu T, Stelmakh L et al (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11(6):521–529
Miller VA, O’Connor P, Soh C et al (2009) A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 27:18s (suppl; abstr LBA8002)
Fidias PM, Dakhil SR, Lyss AP et al (2009) Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 27(4):591–598
Grossi F, Aita M, Follador A et al (2007) Sequential, alternating and maintenance/consolidation chemotherapy in advanced NSCLC: a review of the literature. Oncologist 12(4):451–464
Day RS (1986) Treatment sequencing, asymmetry, and uncertainty: protocol strategies for combination chemotherapy. Cancer Res 46(8):3876–3885
Fossella F, Pereira JR, von Pawel J et al (2003) Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 21(16):3016–3024
Douillard JY, Laporte S, Fossella F et al (2007) Comparison of docetaxel- and vinca alkaloid-based chemotherapy in the first-line treatment of advanced non-small cell lung cancer: a meta-analysis of seven randomized clinical trials. J Thorac Oncol 2(10):939–946
Niho S, Ohe Y, Kakinuma R et al (2002) Phase II study of docetaxel and cisplatin administered as three consecutive weekly infusions for advanced non-small cell lung cancer. Lung Cancer 35(2):209–214
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
National Cancer Institute Common toxicity criteria version 2.0. http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38(1):143–151
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Facchini G, Caraglia M, Morabito A et al (2010) Metronomic administration of zoledronic acid and taxotere combination in castration resistant prostate cancer patients: phase I ZANTE trial. Cancer Biol Ther 10(6):543–548
Edelman MJ, Gandara DR, Lau DH et al (2001) Sequential combination chemotherapy in patients with advanced nonsmall cell lung carcinoma. Cancer 92(1):146–152
Clark JI, Kancharla K, Qamar R et al (2001) Pilot study of sequential vinorelbine and cisplatin followed by docetaxel for selected IIIB and stage IV non-small cell lung cancer. Lung cancer 34(2):271–277
Hosoe S, Komuta K, Shibata K et al (2003) Gemcitabine and vinorelbine followed by docetaxel in patients with advanced non-small-cell lung cancer: a multi-institutional phase II trial of nonplatinum sequential triplet combination chemotherapy (JMTO LC00–02). Br J Cancer 88(3):342–347
Binder D, Schweisfurth H, Grah C et al (2007) Docetaxel/gemcitabine or cisplatin/gemcitabine followed by docetaxel in the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC): results of a multicentre randomized phase II trial. Cancer Chemother Pharmacol 60(1):143–150
Grossi F, Belvedere O, Fasola G et al (2004) Sequential chemotherapy with paclitaxel plus cisplatin followed by vinorelbine, followed by gemcitabine in advanced non-small cell lung cancer: an Alpe Adria Thoracic Oncology Multidisciplinary group study (ATOM 001). Lung Cancer 46(1):99–106
Edelman MJ, Clark JI, Chansky K et al (2004) Randomized phase II trial of sequential chemotherapy in advanced non-small cell lung cancer (SWOG 9806): carboplatin/gemcitabine followed by paclitaxel or cisplatin/vinorelbine followed by docetaxel. Clin Cancer Res 10(15):5022–5026
Novello S, Falcone A, Crinò L et al (2009) Randomised multicenter phase II study of two schedules of docetaxel and gemcitabine or cisplatin/gemcitabine followed by docetaxel as first line treatment for advanced non-small cell lung cancer. Lung Cancer 66(3):327–332
Kubota K, Kawahara M, Ogawara M et al (2008) Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol 9(12):1135–1142
Acknowledgments
The study was supported by Sanofi-Aventis Italy.
Conflict of interest
Francesco Grossi, Filippo de Marinis, Vittorio Gebbia, Ferdinando Riccardi, Orazio Caffo, Teresa Gamucci, Francesco Ferraù, Mario Nardi, Luca Moscetti, Luca Boni, Enzo Galligioni have no conflicts of interest. Davide Dondi is full employee of Sanofi-Aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grossi, F., de Marinis, F., Gebbia, V. et al. A randomised phase II trial of two sequential schedules of docetaxel and cisplatin followed by gemcitabine in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 69, 369–375 (2012). https://doi.org/10.1007/s00280-011-1710-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1710-0