Abstract
Purpose
The objectives of this study were to investigate the pharmacokinetics of intra-venous vinorelbine combined with lapatinib as well as the effect of covariates in breast cancer patients.
Methods
Women with HER2 + locally advanced or metastatic breast cancer progressing after ≤2 lines of trastuzumab-based treatment were treated with lapatinib per os starting 7 days (D) (D-7 to D0) before adding vinorelbine on a D1 & D8 every 3 weeks intravenous schedule. Lapatinib was given everyday. Dose levels [DL, lapatinib (mg)/vinorelbine (mg/m2)] ranged from 750/20 to 1,250/25. A total of 29 patients, 37–76 years old, were treated with the combination of lapatinib + vinorelbine. For pharmacokinetic analysis, 7 time point samples were collected on D1 of cycle 1 for lapatinib and vinorelbine assays. For vinorelbine and lapatinib, respectively, whole blood and plasma concentrations were measured using ultra performance liquid chromatography with tandem mass spectrometry validated methods. Data analysis was performed using a non-linear mixed effect model program (Monolix version 3.1 s).
Results
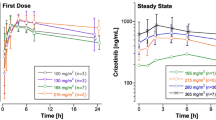
A three-compartment open model adequately described vinorelbine pharmacokinetics. Body weight (BW) and platelet count significantly influenced blood vinorelbine clearance (CL). BW significantly influenced volume (V) and CL terms. Platelet count influenced vinorelbine elimination CL. The final parameter estimates were as follows: CL = 24.9 L/h, V1 = 8.48 L, Q2 = 50.7 L/h, V2 = 1,320 L, Q3 = 66.1 L/h, and V3 = 62.4 L (Qi and Vi denote inter-compartmental clearance and peripheral volume of distribution, respectively), normalized for a 70-kg patient according to BW allometric scaling (CL is normalized for a 250,000 platelet count). A one-compartment model with linear elimination adequately fitted the lapatinib plasma concentration–time data. The population pharmacokinetic parameters were CL = 27.7 L/h, V = 357 L, and the absorption constant, ka = 0.44 h−1. The between-subject variabilities (BSV) could be well estimated for CL, V but not for ka. No covariate effect, including body surface area and vinorelbine dosage, could be identified for lapatinib.
Conclusions
The pharmacokinetic modeling of vinorelbine and lapatinib was consistent with the results previously reported. BW and platelet count were confirmed as influencing blood CL of vinorelbine. A pharmacokinetic interaction occurred between vinorelbine and lapatinib probably due to lapatinib inhibition of CYP450-3A4. The combined lapatinib administration decreases statistically significant the vinorelbine CL. The maximal tolerated dose for the combination of lapatinib with vinorelbine on a q3w schedule is as follows: lapatinib 1,000 mg/day continuously and vinorelbine 22.5 mg/m2 D1 & D8.





Similar content being viewed by others
References
Zhou XJ, Rahmani R (1992) Preclinical and clinical pharmacology of vinca alkaloids. Drugs 44(Suppl 4):1
Gregory RK, Smith IE (2000) Vinorelbine: a clinical review. Br J Cancer 82:1907
Crown JP, Burris HA III, Boyle F, Jones S, Koehler M, Newstat BO, Parikh R, Oliva C, Preston A, Byrne J (2008) Pooled analysis of diarrhea events in patients with cancer treated with lapatinib. Breast Cancer Res Treat 112:317
Gomez HL, Chavez MA, Doval DC, (2006). Results from a phase II randomized study of lapatinib as first-line treatment for patients with ErbB2-amplified advanced or metastatic breast cancer. Breast cancer Res Treat 100:S68. Abstract 1090
Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, Ng C, Franco SX, Chow LW, Arbushites MC, Casey MA, Berger MS, Stein SH, Sledge GW (2008) Efficacy and safety of lapatinib as first-line treatment for patients with ErbB2-amplified advanced or metastatic breast cancer. J Clin Oncol 26:2999
Teng WC, Oh JW, New LS, Wahlin MD, Nelson SD, Ho HK, Chan ECY (2010) Mechanism-Based inactivation of cytochrome P450 3A4 by lapatinib. Mol Pharmacol 78:693
Nguyen L, Tranchand B, Puozzo C, Variol P (2001) Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br J Clin Pharmacol 53:459
Van Heugen JC, De Graeve J, Zorza G, Puozzo C (2001) New sensitive liquid chromatography method coupled with tandem mass spectrometric detection for the clinical analysis of vinorelbine and its metabolites in blood, plasma, urine and faeces. J Chromatogr A 926:11
Bai F, Freeman BB III, Fraga CH (2006) Determination of lapatinib (GW572016) in human plasma by liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS). J Chromatogr B Anal Technol Biomed Life Sci 831:169
Kuhn E et al (2005) Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49:1020–1030
Development Core R, Team R (2009) A language and environment for statistical computing. R Foundation for Statistical Computin. Vienna, Austria
Brendel K et al (2010) Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn 37:49–65
Urien S, Brée F, Breillout F, Bastian G, Krikorian A, Tillement JP (1993) Vinorelbine high-affinity binding to human platelets and lymphocytes: distribution in human blood. Cancer Chemother Pharmacol 32:231
Puozzo C, Gridelli C (2004) Non-small-cell lung cancer in elderly patients: Influence of age on vinorelbine oral pharmacokinetics. Clinical Lung Cancer 237
Khayat D, Rixe O, Brunet R, Goupil A, Bugat R, Harousseau JL, Ifrah N, Puozzo C (2004). Pharmacokinetic linearity of i.v. vinorelbine from an intra-patient dose escalation study design. Cancer Chemother Pharmacol 54:193
Wong M, Balleine RL, Blair EYL, McLachlan AJ, Ackland SP, Grag MB, Evans S, Farlow D, Collins M, Rivory LP, Hoskins JM, Mann GJ, Clarke CL, Gurney H (2006) Predictors of vinorelbine pharmacokinetics and pharmacodynamics in patients with cancer. J Clin Oncol 24:2448
Thiessen B, Stewart C, Tsao M, Kamel-Reid S, Schiaquevich P, Mason W, Belanger K, Forsyth P, McIntosh L, Eisenhauer E (2010) A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol 65:353
Acknowledgments
We acknowledge Federation des Centres de Lutte Contre le Cancer (Groupe d’Etudes Précoces) for their support and contribution into management of this study. We would also like to acknowledge GSK laboratories for their support. We wish to thank Bernard Asselain, Biostatistics Department of Institut Curie for his help.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Rezai and S. Urien contributed equally to this work and are considered as first author.
Rights and permissions
About this article
Cite this article
Rezai, K., Urien, S., Isambert, N. et al. Pharmacokinetic evaluation of the vinorelbine–lapatinib combination in the treatment of breast cancer patients. Cancer Chemother Pharmacol 68, 1529–1536 (2011). https://doi.org/10.1007/s00280-011-1650-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1650-8