Abstract
Background
TMS (2,3′,4,5′-tetramethoxystilbene), a stilbene analog derived from rhapontigenin, was previously demonstrated to induce apoptosis in hormone-resistant breast cancer cells. Therefore, this study investigated the anticancer effect of a new stilbene analog, HTMS ((E)-2-hydroxy-3′,4,5′-trimethoxystilbene), and its mechanism in various breast cancer cell lines.
Materials and methods
The effect of HTMS on cell proliferation of MDA-MB-231, MCF-7, and LTED cells was evaluated using MTT assays. Cell apoptosis was detected by FITC-annexin V staining and flow cytometry analysis, changes in mitochondrial potential were determined by fluorescence microscopy using TMRE staining, and the expression of cleaved PARP and release of cytochrome c were assessed by Western blot analysis.
Results
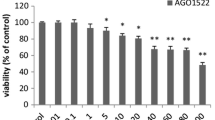
HTMS significantly decreased the cell viability of various types of breast cancer cells in a dose- and time-dependent manner, characterized by G2/M arrest of the cell cycle and the induction of apoptosis. In particular, HTMS disturbed the mitochondrial membrane potential, causing a release of cytochrome c during apoptosis. Furthermore, HTMS was superior to TMS in inhibiting cancer cell growth in a pilot comparison study.
Conclusion
HTMS is an effective apoptotic agent for breast cancer cells, making it a candidate therapeutic agent for the treatment of breast cancer.







Similar content being viewed by others
Abbreviations
- DMSO:
-
Dimethylsulfoxide
- HTMS:
-
(E)-2-Hydroxy-3′,4,5′-trimethoxystilbene
- LTED breast cancer:
-
Long-term estradiol-deprived breast cancer
- MTT:
-
3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazoliumbromide
- PI:
-
Propidium iodide
- Rhapontigenin:
-
3,5,3′-Trihydroxy-4′-methoxy-trans-stilbene
- TMS:
-
2,3′,4,5′-Tetramethoxystilbene
References
Soleas GJ, Goldberg DM, Grass L, Levesque M, Diamandis EP (2001) Do wine polyphenols modulate p53 gene expression in human cancer cell lines? Clin Biochem 34:415–420
Chanvitayapongs S, Draczynska-Lusiak B, Sun AY (1997) Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. Neuroreport 8:1499–1502
Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM (1997) Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci 61:2103–2110
Belleri M, Ribatti D, Nicoli S, Cotelli F, Forti L, Vannini V, Stivala LA, Presta M (2005) Antiangiogenic and vascular-targeting activity of the microtubule-destabilizing trans-resveratrol derivative 3, 5, 4′-trimethoxystilbene. Mol Pharmacol 67:1451–1459
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Bhat KP, Pezzuto JM (2002) Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci 957:210–229
Blumenstein I, Keseru B, Wolter F, Stein J (2005) The chemopreventive agent resveratrol stimulates cyclic AMP-dependent chloride secretion in vitro. Clin Cancer Res 11:5651–5656
Dong Z (2003) Molecular mechanism of the chemopreventive effect of resveratrol. Mutat Res 523–524:145–150
Lu R, Serrero G (1999) Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol 179:297–304
Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R (2003) Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. Faseb J 17:2339–2341
Garvin S, Ollinger K, Dabrosin C (2006) Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett 231:113–122
Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A, Fernandez-Salguero PM (2005) Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer 115:74–84
Alkhalaf M (2007) Resveratrol-induced growth inhibition in MDA-MB-231 breast cancer cells is associated with mitogen-activated protein kinase signaling and protein translation. Eur J Cancer Prev 16:334–341
Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP (2002) Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther 302:369–373
Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM (2002) Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 33:387–398
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr, Walle UK (2004) High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 32:1377–1382
Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, Buscemi F, Grimaudo S, Tolomeo M (2003) Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem 46:3546–3554
Peter Guengerich F, Chun YJ, Kim D, Gillam EM, Shimada T (2003) Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat Res 523–524:173–182
Ovesna Z, Horvathova-Kozics K (2005) Structure-activity relationship of trans-resveratrol and its analogues. Neoplasma 52:450–455
Soares DG, Andreazza AC, Salvador M (2003) Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems. J Agric Food Chem 51:1077–1080
Kim S, Ko H, Park JE, Jung S, Lee SK, Chun YJ (2002) Design, synthesis, and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem 45:160–164
Roupe KA, Remsberg CM, Yanez JA, Davies NM (2006) Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol 1:81–101
Ma Z, Molavi O, Haddadi A, Lai R, Gossage RA, Lavasanifar A (2008) Resveratrol analog trans 3, 4, 5, 4′-tetramethoxystilbene(DMU-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother Pharmacol 63:27–35
Park H, Aiyar SE, Fan P, Wang J, Yue W, Okouneva T, Cox C, Jordan MA, Demers L, Cho H, Kim S, Song RX, Santen RJ (2007) Effects of tetramethoxystilbene on hormone-resistant breast cancer cells: biological and biochemical mechanisms of action. Cancer Res 67:5717–5726
Kim S, Min SY, Lee SK, Cho WJ (2003) Comparative molecular field analysis study of stilbene derivatives active against A549 lung carcinoma. Chem Pharm Bull (Tokyo) 51:516–521
Santen R, Jeng MH, Wang JP, Song R, Masamura S, McPherson R, Santner S, Yue W, Shim WS (2001) Adaptive hypersensitivity to estradiol: potential mechanism for secondary hormonal responses in breast cancer patients. J Steroid Biochem Mol Biol 79:115–125
Dell’Erba C, Chiavarina B, Fenoglio C, Petrillo G, Cordazzo C, Boncompagni E, Spinelli D, Ognio E, Aiello C, Mariggio MA, Viale M (2005) Inhibition of cell proliferation, cytotoxicity and induction of apoptosis of 1, 4-bis (1-naphthyl)-2, 3-dinitro-1, 3-butadiene in gastrointestinal tumour cell lines and preliminary evaluation of its toxicity in vivo. Pharmacol Res 52:271–282
Drummond RM, Mix TC, Tuft RA, Walsh JV Jr, Fay FS (2000) Mitochondrial Ca2+ homeostasis during Ca2+ influx and Ca2+ release in gastric myocytes from Bufo marinus. J Physiol 522(Pt 3):375–390
Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY (2006) Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells. Mol Cancer Ther 5:2034–2042
Billam M, Sobolewski MD, Davidson NE (2009) Effects of a novel DNA methyltransferase inhibitor zebularine on human breast cancer cells. Breast Cancer Res Treat 120:581–592
Zhang N, Kong X, Yan S, Yuan C, Yang Q (2010) Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci 101:2375–2383
Okada H, Mak TW (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 4:592–603
Hirsch T, Marzo I, Kroemer G (1997) Role of the mitochondrial permeability transition pore in apoptosis. Biosci Rep 17:67–76
Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku HH, Murphy MP (2000) Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in Jurkat cells. FEBS Lett 475:267–272
Precht TA, Phelps RA, Linseman DA, Butts BD, Le SS, Laessig TA, Bouchard RJ, Heidenreich KA (2005) The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death Differ 12:255–265
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Gogvadze V, Orrenius S, Zhivotovsky B (2006) Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta 1757:639–647
Scovassi AI, Poirier GG (1999) Poly(ADP-ribosylation) and apoptosis. Mol Cell Biochem 199:125–137
Jeng MH, Shupnik MA, Bender TP, Westin EH, Bandyopadhyay D, Kumar R, Masamura S, Santen RJ (1998) Estrogen receptor expression and function in long-term estrogen-deprived human breast cancer cells. Endocrinology 139:4164–4174
Acknowledgments
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Government (MEST) (No. 2009-0091573).
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. S. Chae and H. J. Jung contributed equally to this article as the first authors.
Rights and permissions
About this article
Cite this article
Chae, Y.S., Kim, J.G., Jung, H.J. et al. Anticancer effect of (E)-2-hydroxy-3′,4,5′-trimethoxystilbene on breast cancer cells by mitochondrial depolarization. Cancer Chemother Pharmacol 68, 349–358 (2011). https://doi.org/10.1007/s00280-010-1464-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1464-0