Abstract
Background
Biliary tract cancers (BTC) have a poor prognosis, and there is no consensus on the best chemotherapy regimen. This study determined the response rate for fixed-dose-rate (FDR) gemcitabine combined with cisplatin.
Methods
This multicentre phase II trial enrolled 50 patients with inoperable locally advanced or metastatic BTC. Treatment consisted of FDR gemcitabine 1,000 mg/m2 (10 mg/m2/min) and cisplatin 20 mg/m2 on days 1 and 8 of a 21-day cycle. The primary endpoint was response rate. Secondary endpoints included safety, response duration (RD), progression-free (PFS) and overall survival (OS), and cancer antigen 19-9 response.
Results
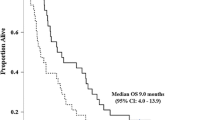
Thirteen patients (26%, 95% CI 14.6–40.4) had a partial response, and 12 (24%) had stable disease. The median RD was 8.3 months (95% CI 6.91–9.99); median PFS 4 months (95% CI 2.5–6.77); and median OS 6.8 months (95% CI 5.0–8.7). Treatment was well tolerated. Grade 3 and grade 4 nausea, vomiting, and fatigue were uncommon. Thirty-eight per cent of patients discontinued treatment because of toxicity, patient or clinician preference.
Conclusions
This treatment combination had moderate activity with acceptable toxicity, supporting previous results that this combination has a role to play. The study does not suggest that FDR gemcitabine is superior to bolus infusion.

Similar content being viewed by others
References
Greenlee RT, Hill-Harmon MB, Murray T et al (2001) Cancer statistics, 2001. CA Cancer J Clin 51:15–36
Patel T (2002) Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2:10
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902
Pasetto LM, D’Andrea MR, Falci C et al (2007) Gemcitabine in advanced biliary tract cancers. Crit Rev Oncol Haematol 61:230–242
Abbruzzese JL, Grunewald R, Weeks EA et al (1991) A phase I clinical, plasma and pharmacology study of gemcitabine. J Clin Oncol 9:491–498
Tempero M, Plunkett W, van Haperen VR et al (2003) Randomised phase II comparison of fixed dose-intense gemcitabine: thirty minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 21:3402–3408
Poplin E, Feng Y, Berlin J et al (2009) Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the eastern cooperative oncology. J Clin Oncol 27:3778–3785
Peters GJ, Bergman AM, Ruiz van Haperen VW et al (1995) Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol 22:72–79
Van Moorsel CJ, Pinedo HM, Veerman G et al (1999) Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small cell lung cancer cell lines. Br J Cancer 80:981–990
Valle JW, Wasan HS, Palmer DD et al (2009) Gemcitabine with or without cisplatin in patients with advanced or metastatic biliary tract cancer: results of a multicenter, randomized phase III trial (the UK ABC-02 trial). J Clin Oncol 27:15s (abstract 4503)
Ko A, Dito E, Schillinger B et al (2006) Phase II study of fixed dose rate gemcitabine with cisplatin for metastatic adenocarcinoma of the pancreas. J Clin Oncol 24:379–385
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Rustin GJ, Nelstrop AE, McClean P et al (1996) Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol 14:1545–1551
Meyerhardt JA, Zhu A, Stuart K et al (2008) Phase II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci 53:564–570
Lee J, Kim TY, Lee MA et al (2008) Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol 61:47–52
Giuliani F, Gebbia V, Maiello E et al (2006) Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicentre phase II study of the Gruppo Oncologica dell’Italia Meridionale. Ann Oncol 17:vii73–vii77
Park BK, Kim YJ, Park JY et al (2006) Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. J Gastronenterol Hepatol 21:993–1003
Kim ST, Park JO, Lee J et al (2006) A phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 106:1339–1346
Thongprasert S, Napapan S, Charoentum C et al (2005) Phase II study of gemcitabine and cisplatin as first line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol 16:279–281
Doval DC, Sekhon JS, Gupta SK et al (2004) A phase II study of gemcitabine and cisplatin in chemotherapy naïve, unresectable gallbladder cancer. Br J Cancer 90:1516–1520
Reyes-Vidal J, Gallardo J, Yanez E et al (2003) Gemcitabine and cisplatin in the treatment of patients with unresectable or metastatic gallbladder cancer: results of the phase II GOCCHI study. Proc Am Soc Clin 22:273 abstract 1095
Lee GW, Kang JH, Kim HG et al (2006) Combination chemotherapy with gemcitabine and cisplatin as first line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol 29:127–131
Ko AH, Hwang J, Venook AP et al (2005) Serum CA19–9 response as a surrogate for clinical outcome in patients receiving fixed dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 93:195–199
Harder J, Kummer O, Olschewski M et al (2007) Prognostic relevance of Carbohydrate Antigen 19.9 levels in patients with advanced biliary tract cancer. Cancer Epidemiol Biomarkers Prev 16:2097–2100
Riechelmann RP, Townsley CA, Chin SN et al (2007) Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 110:1307–1312
Iyer RV, Gibbs J, Kuvshinoff B et al (2007) A phase II study of gemcitabine and capecitabine in advanced cholangiocarcinoma and carcinoma of the gallbladder. A single institution study. Ann Surg Oncol 14:3202–3209
Andre T, Tournigand C, Rosmorduc O et al (2004) Gemcitabine combined with oxaliplatin in advanced biliary tract adenocarcinoma. A GERCOR study. Ann Oncol 15:1339–1343
Verderame F, Russo A, Di Leo R et al (2006) Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Ann Oncol 17(suppl 7):68–72
Park SH, Park YH, Lee JN et al (2006) Phase II study of epirubicin, cisplatin and capecitabine for advanced biliary tract adenocarcinoma. Cancer 106:361–365
Acknowledgments
Numerous individuals from many institutions participated to complete this study. The authors thank everyone involved for their efforts. This work was supported by an unrestricted educational grant from Eli Lilly, a Partnership grant from the Cancer Institute NSW, and by the National Health and Medical Research Council Clinical Trials Centre.
Conflict of interest statement
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the Australasian Gastrointestinal Trials Group.
Trial registration: ACTRN12605000001695.
Rights and permissions
About this article
Cite this article
Goldstein, D., Gainford, M.C., Brown, C. et al. Fixed-dose-rate gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol 67, 519–525 (2011). https://doi.org/10.1007/s00280-010-1351-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1351-8