Abstract
Objectives
Hemorrhagic cystitis (HC) is a major dose-limiting side effect of cyclophosphamide (CP). The mechanism by which CP induces cystitis is not clear. Recent studies demonstrate that nitric oxide; (peroxynitrite) is involved in bladder damage caused by CP. However, the molecular targets of peroxynitrite are not known. The present study is aimed at investigating whether proteins and DNA are molecular targets of peroxynitrite using a rat model.
Methods
The experimental rats received a single i.p. injection of 150 mg kg−1 body weight CP in saline and killed 6 or 16 h later. The control rats received saline. The bladders were used for histological and biochemical analysis. Nitrotyrosine and poly-(ADP-ribose) polymerase (PARP) were localized immunohistochemically as indicators of protein nitration and DNA damage, respectively. Nitrite, malondialdehyde, protein thiol and superoxide dismutase (SOD) activity were assayed in the bladder.
Results
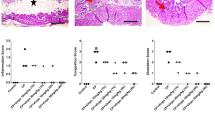
Hematuria and urinary bladder edema was observed in the CP-treated rats and histologically, moderate to severe damage to the urinary bladder was observed. The bladders of CP-treated rats stained strongly for nitrotyrosine as well as for PARP. Significant decrease in oxidized NAD levels was observed in the bladders of CP-treated rats 16 h following treatment with CP. Protein thiol was depleted and the activity of the peroxynitrite sensitive enzyme SOD was significantly reduced in the bladders of CP-treated rats.
Conclusion
The results of the present study reveal that protein nitration, PARP activation and NAD+ depletion may play a critical role in the pathogenesis of CP-induced hemorrhagic cystitis. Based on the results we propose a mechanism for CP-induced cystitis.



Similar content being viewed by others
References
Levine AL, Richie PJ (1989) Urological complications of cyclophosphamide. J Urol 41:1063–1069
West NJ (1997) Prevention and treatment of hemorrhagic cystitis. Pharmacotherapy 4:696–706
Brock N, Pohl J, Stekar J (1981) Studies on the urotoxicity of oxazaphosphorine cytostatics and it prevention. 1. Experimental studies on the urotoxicity of alkylating compounds. Eur J Cancer 7:595–601
Korkmaz A, Oter S, Sadir S et al (2005) Peroxynitrite may be involved in bladder damage caused by cyclophosphamide in rats. J Urol 173:1793–1796
Linares-Fernández BE, Alfieri AB (2007) Cyclophosphamide induced cystitis: role of nitric oxide synthase, cyclooxygenase-1 and 2, and NK (1) receptors. J Urol 177:1531–1536
Radi R, Beckman JS, Bush KM (1991) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288:481
Szabo C, Zingarelli B, O’Connor M et al (1996) DNA strand breakage, activation of poly (ADP-ribose) synthase and cellular energy depletion are involved in the cytotoxicity of macrophages, and smooth muscle cell exposed to peroxynitrite. Proc Natl Acad Sci 93:1753–1758
Radi R (2004) Nitric oxide, oxidants, and protein tyrosine nitration. Proc Nat Acad Sci USA 101:4003–4008
MacMillan-Crow LA, Crow JP, Kerby JD et al (1996) Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA 93:11853–11858
Radons J, Heller B, Burkle A et al (1994) Nitric oxide toxicity in islet cells involve poly (ADP-ribose) polymerase activation and concomitant NAD+ depletion. Biochem Biophys Res Commun 199:1270–1277
Zhang J, Dawson VL, Dawson TM et al (1994) Nitric oxide activation of poly (ADP-ribose) synthetase in neurotoxicity. Science 263:687–689
Zhang J, Pieper A, Snyder SH (1995) Poly (ADP-ribose) synthetase activation: an early indicator of neurotoxic DNA damage. J Neurochem 65:1411–1414
Althaus FR, Richter C (1987) ADP-ribosylation of proteins: enzymology and biological significance. Mol Biol Biochem Biophys 37:1–237
Radi R, Denicola A, Alvarez B et al (2000) In: Ignarro L (ed) Nitric oxide. Academic Press, San Diego, pp 57–82
Ahluwalia A, Maggi CA, Santicioli P et al (1994) Characterization of the capsaicin sensitive component of cyclophosphamide-induced inflammation in the rat urinary bladder. Br J Pharmacol 111:1017–1022
Cuzzocrea S, Zingarelli B, O’Connor M et al (1997) Role of peroxynitrite and activation of poly (ADP-ribose) synthase in the vascular failure induced by zymosan-activated plasma. Br J Pharmacol 122:493–503
Kupper JH, van Gool L, Muller M et al (1996) Detection of poly (ADP-ribose) polymerase and its reaction product by immunohistochemistry. Histochem J 28:391–395
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Ohkuma N, Matsuo S, Tutsui M et al (1982) Superoxide dismutase in the epidermis (author’s transl). Nippon Hifuka Gakkai Zasshi 92:583–590
Sastry KV, Moudgal RP, Mohan J et al (2002) Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Anal Biochem 306:79–82
Matsumura H, Miyachi S (1980) Cycling assay for nicotinamide adenine dinucleotides. Methods Enzymol 69:465–470
Oter S, Korkmaz A, Ostaz E et al (2004) Inducible nitric oxide synthase inhibition in cyclophosphamide induced hemorrhagic cystitis. Urol Res 32:185
Topal T, Oztas Y, Korkmaz A et al (2005) Melatonin ameliorates bladder damage induced by cyclophosphamide in rats. J Pineal Res 38:272–277
Stamler JS (1992) S nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA 89:444–448
Cosi C, Suzuki H, Milani D et al (1992) Poly (ADP-ribose) polymerase: early involvement in glutamate-induced neurotoxicity in cultured cerebellar granule cells. J Neurosci Res 39:38–46
Zingarelli B, O’Conner M, Wong H et al (1996) Peroxynitrite mediated DNA strand breakage activates polyadenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol 156:350–358
Nosseri C, Coppola S, Ghibelli L (1992) Possible involvement of poly (ADP-ribosyl) polymerase in triggering stress-induced apoptosis. Exp Cell Res 212:367–373
Korkmaz A, Topal T, Oter S (2007) Pathophysiological aspects of cyclophosphamide and isofamide induced hemorrhagic cystitis; implications of reactive oxygen species as well as PARP activation. Cell Biol Toxicol 23:303–312
de la Lastra CA, Villegas I, Sánchez-Fidalgo S (2007) Poly (ADP-ribose) polymerase inhibitors: new pharmacological functions and potential clinical implications. Curr Pharm Res 13:933–962
Korkmaz A, Kurt B, Yildirim I, Basal S, Topal T, Sadir S, Oter S (2008) Effects of poly(ADP-ribose) polymerase inhibition caused by cyclophosphamide in rats. Exp Biol Med (Maywood) 233:338–343
Viralag L, Szabo C (2002) The therapeutic potential of poly (ADP-Ribose) polymerase inhibitors. Pharmacol Rev 54:375
Acknowledgments
The project is funded by Department of Science and Technology (DST), India. We thank Dr. K. Indirani for her assistance in light microscopic studies and Ms K. Preethi for her technical assistance in biochemical studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abraham, P., Rabi, S. Protein nitration, PARP activation and NAD+ depletion may play a critical role in the pathogenesis of cyclophosphamide-induced hemorrhagic cystitis in the rat. Cancer Chemother Pharmacol 64, 279–285 (2009). https://doi.org/10.1007/s00280-008-0868-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0868-6