Abstract
Purpose
Irinotecan at 180 mg/m² combined with an infusional 5-fluorouracil/leucovorin (5-FU/LV) regimen (FOLFIRI) is a standard first line therapy for metastatic colorectal cancer (mCRC). This phase II study aimed to assess whether increasing the irinotecan dose in the first line FOLFIRI regimen would benefit mCRC patients.
Patients and methods
Patients received FOLFIRI every 2 weeks for up to six cycles, comprising a 5-FU/LV regimen combined with irinotecan at 180 mg/m² (cycle 1), increasing to 220 mg/m² (cycle 2) and 260 mg/m² (cycle 3 and subsequent cycles) dependent on toxicity. Efficacy and safety were determined in the intention to treat (ITT) population and in patients able to receive irinotecan at 260 mg/m² for at least four cycles [high-dose (HD) population].
Results
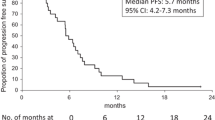
Fifty-four eligible patients were included. Among them, 44 (81.5%) formed the HD population. The ITT objective response rate was 48% (90%CI: 36–60) with 25/26 of the responses in the HD population. The disease control rate was 76% (90%CI: 65–85) and median overall survival was 20.4 months (90%CI: 6.4–27.1). The main grade 3/4 toxicities (ITT/HD populations) were neutropenia (61%/59%), and diarrhoea (18%/11%), respectively.
Conclusions
This study confirms the feasibility of increasing the standard dose of the irinotecan component of FOLFIRI to 260 mg/m², for more than 80% of patients but does not support a clear advantage of this strategy on unselected mCRC patients.
Similar content being viewed by others
References
(1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis group in cancer. J Clin Oncol 16:301–308
Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60:6921–6926
Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. Ann Oncol 16:481–488
de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, Morvan F, Louvet C, Guillot T, Francois E, Bedenne L (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15:808–815
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041–1047
Ducreux M, Ychou M, Seitz JF, Bonnay M, Bexon A, Armand JP, Mahjoubi M, Mery-Mignard D, Rougier P (1999) Irinotecan combined with bolus fluorouracil, continuous infusion fluorouracil, and high-dose leucovorin every two weeks (LV5FU2 regimen): a clinical dose-finding and pharmacokinetic study in patients with pretreated metastatic colorectal cancer. J Clin Oncol 17:2901–2908
Duffour J, Gourgou S, Seitz JF, Senesse P, Boutet O, Castera D, Kramar A, Ychou M (2002) Efficacy of prophylactic anti-diarrhoeal treatment in patients receiving Campto for advanced colorectal cancer. Anticancer Res 22:3727–3731
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics, 2006. CA Cancer J Clin 56:106–130
Kohne CH, van Cutsem E, Wils J, Bokemeyer C, El-Serafi M, Lutz MP, Lorenz M, Reichardt P, Ruckle-Lanz H, Frickhofen N, Fuchs R, Mergenthaler HG, Langenbuch T, Vanhoefer U, Rougier P, Voigtmann R, Muller L, Genicot B, Anak O, Nordlinger B (2005) Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol 23:4856–4865
Poujol S, Bressolle F, Duffour J, Abderrahim AG, Astre C, Ychou M, Pinguet F (2006) Pharmacokinetics and pharmacodynamics of irinotecan and its metabolites from plasma and saliva data in patients with metastatic digestive cancer receiving Folfiri regimen. Cancer Chemother Pharmacol 58:292–305
Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C (1998) Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352:1407–1412
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Van Cutsem E, Dirix L, Van Laethem JL, Van Belle S, Borner M, Gonzalez Baron M, Roth A, Morant R, Joosens E, Gruia G, Sibaud D, Bleiberg H (2005) Optimisation of irinotecan dose in the treatment of patients with metastatic colorectal cancer after 5-FU failure: results from a multinational, randomised phase II study. Br J Cancer 92:1055–1062
Ychou M, Raoul JL, Desseigne F, Borel C, Caroli-Bosc FX, Jacob JH, Seitz JF, Kramar A, Hua A, Lefebvre P, Couteau C, Merrouche Y (2002) High-dose, single-agent irinotecan as first-line therapy in the treatment of metastatic colorectal cancer. Cancer Chemother Pharmacol 50:383–391
Acknowledgments
This work was supported in part by Regional PHRC (Programme Hospitalier de Recherche Clinique), in part by Laboratoire Chugaï.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duffour, J., Gourgou, S., Desseigne, F. et al. Multicentre phase II study using increasing doses of irinotecan combined with a simplified LV5FU2 regimen in metastatic colorectal cancer. Cancer Chemother Pharmacol 60, 383–389 (2007). https://doi.org/10.1007/s00280-006-0372-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0372-9