Abstract
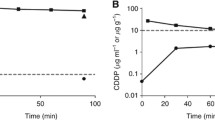
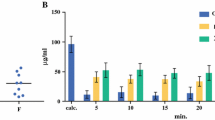
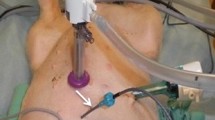
Background: The rationale supporting the use of intraperitoneal chemotherapy in peritoneal surface malignancy relates to a large local–regional effect and low systemic toxicity. While optimizing the use of this treatment strategy, little information regarding the effect of volume of chemotherapy solution is available. Objective: The goal of this study was to provide data regarding the effect of volume of chemotherapy solution on the pharmacokinetics of intraperitoneal chemotherapy. Data by which to optimally adjust this parameter during intraperitoneal chemotherapy treatments were sought. Methods: Forty-eight patients with peritoneal surface malignancy were treated with hyperthermic intraperitoneal mitomycin C chemotherapy after a complete cytoreduction to remove all visible evidence of mucinous tumor. The dose of mitomycin C was always 12.5 mg/m2 in males and 10 mg/m2 in females. The first 12 patients were treated with 6 l of 1.5% dextrose peritoneal dialysis solution. The next 14 patients were treated with 4 l of fluid and then ten patients were treated with 2 l. In the last 12 patients the volume of fluid was 1.5 l/m2 . Blood, peritoneal fluid, and urine samples were obtained every 15 min for 90 min; additional blood and urine samples were obtained at 120 min. Mitomycin C concentrations, urine volumes, and final intraperitoneal fluid volume were obtained. Results: The intraperitoneal and the plasma concentrations were highest in the 2-l group, less in the 4-l group, and least in the 6-l group. All differences were statistically significant. Also, the percent of mitomycin C absorbed decreased significantly from 2, to 4, to 6 l of fluid. The area under the curve (AUC) ratio of intraperitoneal concentration times time to intravenous concentration times time was 27.01±4.92 for 2 l, 22.22±7.95 for 4 l, and 24.01±8.46 for 6 l. These differences were not statistically significant. If both the volume of chemotherapy solution and the total dose of mitomycin C were determined from the body surface area, the pharmacokinetics of intraperitoneal mitomycin C were more consistent. Conclusions: In order to prescribe a uniform treatment for patients receiving hyperthermic intraperitoneal mitomycin C, the total dose of the drug and the total volume of chemotherapy solution should be determined from the body surface area. If the volume of chemotherapy solution is not based on patient body surface area, predictions regarding toxicity are less precise.




Similar content being viewed by others
References
Yu W, Whang I, Suh I, Averbach A, Chang D, Sugarbaker PH (1998) Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg 228:347–354
Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH (2001) Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg 25:985–990
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FAN (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21:3737–3743
Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AAK, Lorimier G, Bernard JL, Bereder JM, Porcheron J, Gomez-Portilla A, Shen P, Deraco M, Rat P (2004) Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer. A multi-institutional study. J Clin Oncol 22:3284–3292
Sugarbaker PH (2004) Managing the peritoneal surface component of gastrointestinal cancer. Part 1. Patterns of dissemination and treatment options. Oncology (Huntingt) 18:51–59
Sugarbaker PH (2004) Managing the peritoneal surface component of gastrointestinal cancer. Part 2. Perioperative intraperitoneal chemotherapy. Oncology (Huntingt) 18:207–219
Sugarbaker PH, Jablonski KA (1995) Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 221:124–132
Sugarbaker PH (1999) Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 6:727–731
Sugarbaker PH (1995) Peritonectomy procedures. Ann Surg 221:29–42
Sugarbaker PH, Averbach AM, Jacquet P, Stephens AD, Stuart OA (1996) A simplified approach to hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) using a self retaining retractor. In: Sugarbaker PH (ed) Peritoneal carcinomatosis: principles of management. Kluwer, Boston, pp 415–421
Eksborg S, Ehrsson H, Lindfors A (1983) Liquid chromatographic determination of mitomycin C in human plasma and urine. J Chromatogr 274:263–270
Tjaden UR, de Bruijn EA, van der Hoeven RA, Jol C, van der Greef J, Lingeman H (1987) Automated analysis of mitomycin C in body fluids by high-performance liquid chromatography with on-line sample pre-treatment. J Chromatogr 420:53–62
Jacquet P, Sugarbaker PH (1996) Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 15:49–58
Ronnet BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM (1995) Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: a clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 19:1390–1408
Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, Steves MA, Sugarbaker PH (1999) Morbidity and mortality of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the Coliseum technique. Ann Surg Oncol 6:790–796
Van Ruth S, Verwaal VJ, Zoetmulder FAN (2003) Pharmacokinetics of intraperitoneal mitomycin C. Surg Oncol Clin N Am 12:771–780
Elias DM, Sideris L (2003) Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg Oncol Clin N Am 12:755–769
De Lima Vazquez V, Stuart OA, Sugarbaker PH (2003) Extent of parietal peritonectomy does not change intraperitoneal chemotherapy pharmacokinetics. Cancer Chem Pharmacol 52:108–112
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugarbaker, P.H., Stuart, O.A. & Carmignani, C.P. Pharmacokinetic changes induced by the volume of chemotherapy solution in patients treated with hyperthermic intraperitoneal mitomycin C. Cancer Chemother Pharmacol 57, 703–708 (2006). https://doi.org/10.1007/s00280-005-0074-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0074-8