Abstract
Purpose
Imatinib (Glivec) has been established as a highly effective therapy for chronic myeloid leukemia and gastrointestinal tumors. The recommended daily dosage of 400–600 mg requires simultaneous intake of up to six of the current 100-mg capsules. Due to the need to swallow multiple capsules per dose, there is a potential negative impact on treatment adherence; therefore, a new imatinib 400-mg film-coated tablet has been developed. To improve dosing flexibility, particularly with regard to the pediatric population and the management of adverse events, a scored 100-mg film-coated tablet has also been introduced.
Experimental design
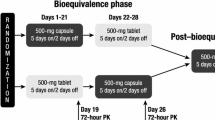
A group of 33 healthy subjects were randomly assigned to one of six treatment sequences, in which they received imatinib as 4×100-mg capsules (reference), 4×100-mg scored tablets (test), and 1×400-mg tablet (test). Blood sampling was performed for up to 96 h after dosing, followed by a 10-day washout period prior to the next sequence. After the third dosing, subjects were monitored to assess delayed drug-related adverse events. Pharmacokinetic parameters were assessed using concentration-time curves for plasma imatinib and its metabolite CGP74588.
Results
Median Tmax was 2.5 h for capsules and tablets. Mean AUC(0–inf) values were 27094, 26081 and 25464 ng·h/ml for 4×100-mg capsules, 4×100-mg tablets, and 1×400-mg tablets, respectively. Cmax values were 1748, 1638 and 1606 ng/ml, and t1/2 values were 15.8, 15.9 and 15.7 h. The test/reference ratios for AUC(0–inf), AUC(0–96 h), and Cmax were 0.98, 0.98 and 0.95 for 4×100-mg tablets versus 4×100-mg capsules, and 0.95, 0.95 and 0.92 for 1×400-mg tablet versus 4×100-mg capsules. The 95% confidence intervals were fully contained within the interval (0.80, 1.25). Eight mild and one moderate adverse event considered to be drug related were reported. These events showed no clustering by type of dosage form and were of little to no clinical significance.
Conclusions
Film-coated 100-mg (scored) and 400-mg tablet dose forms of imatinib are bioequivalent to the commercial 100-mg hard-gelatin capsule, and are as safe and well tolerated.


Similar content being viewed by others
References
Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB (2000) Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-Kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295:139
Peggs K, Mackinnon S (2003) Imatinib mesylate—the new gold standard for treatment of chronic myeloid leukemia. N Engl J Med 348:1048
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantargian H, Capdeville R, Ohno-Jones O, Sawyers CL (2001) Efficacy and safety of a specific inhibitor of the Bcr-Abl tyrosine kinase in chronic myeloid leukemia. New Engl J Med 344:1031
Deininger MWN, O’Brien SG, Ford JM, Druker BJ (2003) Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol 21:1637
Partridge AH, Avorn J, Wang PS, Winer EP (2002) Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94:652
Verweij J (2003) Early efficacy comparison of two doses of imatinib for the treatment of advanced gastro-intestinal stromal tumors (GIST): interim results of a randomized phase III trial from the EORTC-STBGS, ISG and AGITG (abstract 3272). Proc Am Soc Clin Oncol 22:814
Novartis Pharma (2001) GlivecTM (imatinib mesylate) (prescribing information). Novartis Pharma, Basel, Switzerland
Bakhtiar R, Lohne J, Ramos L, Khemani L, Hayes M, Tse F (2002) High-throughput quantification of the anti-leukemia drug STI571 (Gleevec) and its main metabolite (CGP 74588) in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 768:325
Dunnett CW (1955) A multiple comparison procedure for comparing several treatments with a control. JASA 50:1096
US Department of Health and Human Services (2003) Guidance for industry. Statistical approaches to establishing bioequivalence. Food and Drug Administration, Office of Training and Communications, Rockville, MD. http://www.fda.gov/cder/guidance/3616fnl.htm
Wilkinson GR (1996) Cytochrome P4503A (CYP3A) metabolism: prediction of in vivo activity in humans. J Pharmacokinet Biopharm 24:475
Demetri GD, von Mehren M, Blanke CD, van den Abeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corles C, Fletcher CD, Joensuu H (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472
O’Brien SGO, Guilhot F, Larson R, Gathmann I, Baccarani M, Cervantes F, Cornelisson JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic phase chronic myeloid leukemia. N Engl J Med 348:994
Acknowledgements
We wish to thank members of the nursing and research staff who participated in this study. The support of Petra Brinkmann, Ann E. Bolton, Agnes Brunner, and Roland Waite at Novartis is gratefully acknowledged. All experimental procedures performed in this study were in accordance with current Swiss law.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikolova, Z., Peng, B., Hubert, M. et al. Bioequivalence, safety, and tolerability of imatinib tablets compared with capsules. Cancer Chemother Pharmacol 53, 433–438 (2004). https://doi.org/10.1007/s00280-003-0756-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0756-z