Abstract
Purpose. Docetaxel (Taxotere) has been shown to possess a broad spectrum of antitumor activity against various malignancies such as breast and lung cancers, but also against intraabdominal malignancies such as mesothelioma and ovarian cancer. For cancers occurring within the abdominal cavity, the advantage of intraperitoneal chemotherapy is the prolonged high drug concentration that can be achieved locally with low systemic toxicity. Using a rat model, this study was designed to compare the pharmacokinetics and tissue distribution of intraperitoneal versus intravenous docetaxel.
Methods. The study animals were comprised of 15 Sprague Dawley rats. They were randomized into three groups according to dose and route of administration (15 mg/kg intravenously, 15 mg/kg intraperitoneally, or 150 mg/kg intraperitoneally) and then given a single dose of docetaxel. Blood and peritoneal fluid were sampled using a standardized protocol for 90 min. At the end of the procedure the rats were killed and docetaxel concentrations in peritoneal fluid, plasma and selected tissue samples were determined by high-performance liquid chromatography (HPLC).
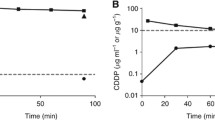
Results. When docetaxel was delivered at 15 mg/kg the area under the curve (AUC) of the peritoneal fluid was significantly higher with intraperitoneal administration (110.6 µg/ml·min) as compared to intravenous administration (0.043 µg/ml·min; P=0.0079). This represents more than a 2500-fold increase in exposure for tissues at peritoneal surfaces after intraperitoneal administration. Conversely, at the same dose the AUC of the plasma was significantly lower with intraperitoneal administration (0.11 µg/ml·min) as compared to intravenous administration (4.25 µg/ml·min; P=0.0079). The AUC ratio (AUC peritoneal fluid/AUC plasma) was 976 for intraperitoneal administration as opposed to 0.01 for intravenous delivery. The AUC ratio for intraperitoneal docetaxel at 150 mg/kg was 3004. There were significantly different concentrations in the heart and the abdominal wall (P=0.0079) and in the stomach and colon (P=0.0159) when intraperitoneal versus intravenous docetaxel were compared.
Conclusions. The exposure of the peritoneal surface to docetaxel is significantly increased and the systemic exposure decreased with intraperitoneal docetaxel administration. Also, high concentrations of drug were observed in the abdominal wall and in the colon after intraperitoneal delivery. This experiment suggests the need for clinical studies to evaluate intraperitoneal administration of docetaxel in humans.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Marchettini, P., Stuart, A.O., Mohamed, F. et al. Docetaxel: pharmacokinetics and tissue levels after intraperitoneal and intravenous administration in a rat model. Cancer Chemother Pharmacol 49, 499–503 (2002). https://doi.org/10.1007/s00280-002-0439-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00280-002-0439-1