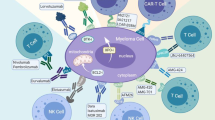
Abstract
From a historic lens, treatment for patients with relapsed/refractory multiple myeloma (R/R MM) has advanced significantly since the advent of immunomodulatory agents (IMiDs) in the 1990s, proteasome inhibitors in the 2000s, monoclonal antibodies in the 2010s, and CAR-T treatments in the 2020s. However, the availability of multiple new therapies has also created significant ambiguity regarding therapy selection and sequencing, as consensus guidelines are limited, and cross-trial comparisons of the novel agents are challenging. In this focused review, we discuss the novel Food & Drug Administration (FDA)-approved medications for R/R MM, including the recently approved first-in-class BCMA-directed bispecific antibody teclistamab. We highlight the seminal clinical trials data and discuss optimal sequencing considerations based on the goal of treatment, with an emphasis on the two novel CAR-T cell products. We consider the limited tolerability of certain agents, prospects for our aging population, and financial aspects of these therapies. Finally, we spotlight ongoing trials involving promising agents making their way through the pharmacologic pipeline including the BCMA-directed bispecific antibody elranatamab and the GPRC5D-directed bispecific antibody talquetamab. We summarize our recommendations based on the best available evidence as we enter 2023.

Similar content being viewed by others
References
National Cancer Institute. Cancer Stat Facts: Myeloma. 2022. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed 10 Nov 2022
Cowan AJ, Green DJ, Kwok M et al (2022) Diagnosis and management of multiple myeloma: a review. JAMA 327:464–477
Munshi NC, Anderson LD, Shah N et al (2021) Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384:705–716
Berdeja JG, Madduri D, Usmani SZ et al (2021) Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398:314–324
Raje N, Berdeja J, Lin Y et al (2019) Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 380:1726–1737
Anderson JLD, Munshi NC, Shah N et al (2021) Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: Updated KarMMa results. J Clin Oncol 39(15_suppl):8016–8016
Martin T, Usmani S, Berdeja J et al (2021) Updated results from CARTITUDE-1: phase 1b/2 study of ciltacabtagene autoleucel, a B-cell maturation antigen–directed chimeric antigen receptor t cell therapy, in patients with relapsed/refractory multiple myeloma. Blood 704:549
Martin T, Usmani SZ, Berdeja JG et al (2022) Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. https://doi.org/10.1200/JCO.22.00842
Usmani SZ, Martin TG, Berdeja JG et al (2022) Phase 1b/2 study of ciltacabtagene autoleucel, a BCMA-directed CAR-T cell therapy, in patients with relapsed/refractory multiple myeloma (CARTITUDE-1): two years post-LPI. J Clin Oncol 40(16_suppl):8028–8028
van de Donk N, Richardson PG et al (2018) CD38 antibodies in multiple myeloma: back to the future. Blood 131:13–29
Overdijk MB, Verploegen S, Bögels M et al (2015) Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311–320
de Weers M, Tai YT, van der Veer MS et al (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840–1848
Palumbo A, Chanan-Khan A, Weisel K et al (2016) Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754–766
Dimopoulos MA, Oriol A, Nahi H et al (2016) Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319–1331
Attal M, Richardson PG, Rajkumar SV et al (2019) Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet 394:2096–2107
Moreau P, Dimopoulos MA, Mikhael J et al (2021) Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet 397:2361–2371
Tai YT, Acharya C, An Z et al (2016) APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 127:3225–3236
Usmani SZ, Garfall AL, van de Donk N et al (2021) Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet 398:665–674
Lonial S, Lee HC, Badros A et al (2020) Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207–221
Usmani S, Alonso A, Quach H et al (2022) P14: DREAMM-9: phase I study of belantamab mafadotin plus standard of care in patients with transplant-ineligible newly diagnosed multiple myeloma. HemaSphere 6:19–19
Usmani SZ, Alonso AA, Quach H et al (2021) DREAMM-9: phase I study of belantamab mafodotin plus standard of care in patients with transplant-ineligible newly diagnosed multiple myeloma. Blood Suppl 1:2738
Moreau P, Garfall AL, van de Donk N et al (2022) Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med 387:495–505
Martiniani R, Di Loreto V, Di Sano C et al (2012) Biological activity of lenalidomide and its underlying therapeutic effects in multiple myeloma. Adv Hematol 842945. https://doi.org/10.1155/2012/842945
Thomas SK, Richards TA, Weber DM (2007) Lenalidomide in multiple myeloma. Best Pract Res Clin Haematol 20:717–735
Kronke J, Udeshi ND, Narla A et al (2014) Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343:301–305
Richardson PG, Oriol A, Beksac M et al (2019) Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol 20:781–794
Chari A, Suvannasankha A, Fay JW et al (2017) Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 130:974–981
Engelhardt M, Waldschmidt JM, Wäsch R (2020) Proteasome inhibition: the dawn of novel therapies in multiple myeloma. Haematologica 107:1018–1019
Richardson PG, Sonneveld P, Schuster MW et al (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352:2487–2498
Dimopoulos MA, Moreau P, Palumbo A et al (2016) Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 17:27–38
Stewart AK, Rajkumar SV, Dimopoulos MA et al (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372:142–152
Mateos MV, Masszi T, Grzasko N et al (2017) Impact of prior therapy on the efficacy and safety of oral ixazomib-lenalidomide-dexamethasone vs. placebo-lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in TOURMALINE-MM1. Haematologica 102:1767–1775
Wang Y, Sanchez L, Siegel DS et al (2016) Elotuzumab for the treatment of multiple myeloma. J Hematol Oncol 9:55
Tai YT, Dillon M, Song W et al (2008) Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 112:1329–1337
Lonial S, Dimopoulos M, Palumbo A et al (2015) ELOQUENT-2 investigators. elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 373:621–31
Dimopoulos MA, Lonial S, White D et al (2020) (2020) Elotuzumab, lenalidomide, and dexamethasone in RRMM: final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J 10:1–10
Dimopoulos MA, Dytfeld D, Grosicki S et al (2018) Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med 379:1811–1822
Azizian NG, Li Y (2020) XPO1-dependent nuclear export as a target for cancer therapy. J Hematol Oncol 13:1–9
Vogl DT, Dingli D, Cornell RF et al (2018) Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol 36:859–866
Chari A, Vogl DT, Gavriatopoulou M et al (2019) Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381:727–738
Grosicki S, Simonova M, Spicka I et al (2020) Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. The Lancet 396:1563–1573
White D, LeBlanc R, Venner C et al (2019) Safety and efficacy of the combination of selinexor, lenalidomide and dexamethasone (SRd) in patients with relapsed/refractory multiple myeloma (RRMM). 17th International Myeloma Workshop 2019; Abstract 353
Durie BGM, Hoering A, Abidi MH et al (2017) Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 389:519–527
Richardson PG, Jacobus SJ, Weller EA et al (2022) Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med 387:132–147
Holstein SA, Jung SH, Richardson PG et al (2017) Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol 4:e431–e442
Facon T, Kumar S, Plesner T et al (2019) Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 380:2104–2115
Roussel M, Lauwers-Cances V, Wuilleme S et al (2021) Up-front carfilzomib, lenalidomide, and dexamethasone with transplant for patients with multiple myeloma: the IFM KRd final results. Blood 138:113–121
Mateos MV, Cavo M, Blade J et al (2020) Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet 395:132–141
Sebag M, Raje NS, Bahlis NJ et al (2021) Elranatamab (PF-06863135), a B-cell maturation antigen (BCMA) targeted CD3-engaging bispecific molecule, for patients with relapsed or refractory multiple myeloma: results from magnetismm-1. Blood 653:895
Lesokhin AM, Arnulf B, Niesvizky R (2022) Initial safety results for MagnetisMM-3: a phase 2 trial of elranatamab, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients (pts) with relapsed/refractory (R/R) multiple myeloma (MM). J Clin Oncol 40(16):8006–8006
Bjorklund CC, Kang J, Amatangelo M et al (2020) Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia 34:1197–1201
Richardson PG, Ocio E, Raje NS et al (2021) CC-92480, a potent, novel cereblon E3 ligase modulator (CELMoD) agent, in combination with dexamethasone (DEX) and bortezomib (BORT) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): preliminary results from the phase 1/2 study CC-92480-MM-002. Blood 138(Supplement 1):2731
Funding
SAP receives research funding from the UMass Center for Clinical and Translational Science (CCTS) Pilot Project Program grant (NIH/NCATS Grant UL1TR001453).
Author information
Authors and Affiliations
Contributions
BT, TM, and SAP wrote the manuscript and gave final approval.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
SAP served on the Multiple Myeloma Advisory Board for Pfizer and serves on the Acute Myeloid Leukemia Advisory Board for Bristol Myers Squibb. SAP has been a consultant for the Dedham Group, SIS International Research, and Adivo Associates.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanenbaum, B., Miett, T. & Patel, S.A. The emerging therapeutic landscape of relapsed/refractory multiple myeloma. Ann Hematol 102, 1–11 (2023). https://doi.org/10.1007/s00277-022-05058-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-05058-5