Abstract
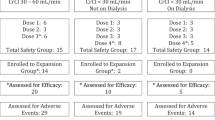
Patients with multiple myeloma (MM) and severe renal impairment (SRI) have shorter survival than MM patients without renal failure. Although lenalidomide is a highly active drug, this immunomodulatory agent is frequently neglected in this context due to its predominant renal clearance and, consequently, an increased risk of toxicity. This risk might be overcome with the proper lenalidomide dose adjustment to renal function. This study evaluates the outcomes of 23 relapsed MM patients with SRI (baseline creatinine clearance (CrCl) <30 mL/min) treated with lenalidomide-dexamethasone (LenDex), including 56 % (13 patients) under hemodialysis. The median CrCl at start of LenDex was 19 mL/min; an overall response rate (partial response or better) of 56 % was obtained, with a median follow-up from start of LenDex of 52 months (8–79). The median time until maximal response was 4 months, and in 58 % (7/12), the response was longer than 2 years. Nine percent had renal improvement, but all the 13 patients on hemodialysis remained under treatment. LenDex was interrupted in three cases because of adverse events (infections and cutaneous events); 78 % of the patients were on thromboprophylaxis with aspirin. It is important to notice that, after initial dose adjustment of therapy, there should be a continuous process of dose adjustment, taking into account variations in renal function. Furthermore, lenalidomide dose adjustment should be made according to the individual tolerance, even with stable renal function. LenDex dose adjustment, according to these principles, does not negatively impact response and improves treatment tolerance. It has a clear potential to treat this group of patients and to induce long duration of responses [event-free survival (EFS) 20.5 m and overall survival (OS) 42.6 m].
Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J Clin 62:10–29. doi:10.3322/caac.20138
Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. Ann Oncol 16:481–8. doi:10.1093/annonc/mdi098
Rachet B, Mitry E, Shah A et al (2008) Survival from multiple myeloma in England and Wales up to 2001. Br J Cancer 99(Suppl 1):S110–2. doi:10.1038/sj.bjc.6604607
Knudsen LM, Hippe E, Hjorth M et al (2009) Renal function in newly diagnosed multiple myeloma—a demographic study of 1353 patients. Eur J Haematol 53:207–212. doi:10.1111/j.1600-0609.1994.tb00190.x
Eleutherakis-Papaiakovou V, Bamias A, Gika D et al (2007) Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma 48:337–41. doi:10.1080/10428190601126602
Knudsen LM, Hjorth M, Hippe E (2000) Renal failure in multiple myeloma: reversibility and impact on the prognosis. Eur J Haematol 65:175–181. doi:10.1034/j.1600-0609.2000.90221.x
Bladé J, Fernández-Llama P, Bosch F et al (1998) Renal failure in multiple myeloma. Arch Intern Med 158:1889. doi:10.1001/archinte.158.17.1889
Gaballa MR, Laubach JP, Schlossman RL et al. (2012) Management of myeloma-associated renal dysfunction in the era of novel therapies
Wirk B (2011) Renal failure in multiple myeloma: a medical emergency. Bone Marrow Transplant 46:771–83. doi:10.1038/bmt.2011.8
Tsakiris DJ, Stel VS, Finne P et al (2010) Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: an ERA-EDTA Registry study. Nephrol Dial Transplant 25:1200–6. doi:10.1093/ndt/gfp679
Dimopoulos MA, Terpos E, Chanan-Khan A et al (2010) Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 28:4976–4984. doi:10.1200/JCO.2010.30.8791
Durie BGM, Harousseau J-L, Miguel JS et al (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–73. doi:10.1038/sj.leu.2404284
Neves M, Martins H, Esteves G, et al. Insuficiência renal à apresentação não é um factor prognóstico adverso em doentes com mieloma múltiplo tratados com novos agentes. Port J Nephrol Hypertens 28:318–324.
Eleftherakis-Papapiakovou E, Kastritis E, Roussou M et al (2011) Renal impairment is not an independent adverse prognostic factor in patients with multiple myeloma treated upfront with novel agent-based regimens. Leuk Lymphoma 52:2299–303. doi:10.3109/10428194.2011.597906
Chertow GM, Burdick E, Honour M et al (2005) Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16:3365–3370. doi:10.1681/ASN.2004090740
Hoste EAJ, Clermont G, Kersten A et al (2006) RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10:R73. doi:10.1186/cc4915
Botev R, Mallié JP, Couchoud C et al (2009) Estimating glomerular filtration rate: Cockcroft-Gault and modification of diet in renal disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol 4:899–906. doi:10.2215/CJN.05371008
Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23:3–9. doi:10.1038/leu.2008.291
Defronzo RA, Humphrey RL, Wright JR, Cooke CR (1975) Acute renal failure in multiple myeloma. Medicine (Baltimore) 54:209–223
Kyle RA (1975) Multiple myeloma: review of 869 cases. Mayo Clin Proc 50:29–40
Sanders PW (1994) Pathogenesis and treatment of myeloma kidney. J Lab Clin Med 124:484–488
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Found. Stat. Comput. Vienna, Austria
Jagannath S, Barlogie B, Berenson JR et al (2005) Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impaired renal function. Cancer 103:1195–1200. doi:10.1002/cncr.20888
San-Miguel JF, Richardson PG, Sonneveld P et al (2008) Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leuk Off J Leuk Soc Am Leuk Res Fund UK 22:842–849. doi:10.1038/sj.leu.2405087
Morabito F, Gentile M, Ciolli S et al (2010) Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: a retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol 84:223–228. doi:10.1111/j.1600-0609.2009.01385.x
Fouquet G, Tardy S, Demarquette H et al (2013) Efficacy and safety profile of long-term exposure to lenalidomide in patients with recurrent multiple myeloma. Cancer 119:3680–3686. doi:10.1002/cncr.28274
Dimopoulos MA, Terpos E, Goldschmidt H et al (2012) Treatment with lenalidomide and dexamethasone in patients with multiple myeloma and renal impairment. Cancer Treat Rev 38:1012–1019. doi:10.1016/j.ctrv.2012.02.009
Weber DM, Chen C, Niesvizky R et al (2007) Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. doi:10.1056/NEJMoa070596
Dimopoulos M, Spencer A, Attal M et al (2007) Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357:2123–32. doi:10.1056/NEJMoa070594
Dimopoulos M, Alegre A, Stadtmauer EA et al (2010) The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer 116:3807–3814. doi:10.1002/cncr.25139
Alegre A, Aguado B, Giraldo P et al (2011) Lenalidomide is effective as salvage therapy in refractory or relapsed multiple myeloma: analysis of the Spanish compassionate use registry in advanced patients. Int J Hematol 93:351–360. doi:10.1007/s12185-011-0785-z
Oehrlein K, Langer C, Sturm I et al (2012) Successful treatment of patients with multiple myeloma and impaired renal function with lenalidomide: results of 4 German centers. Clin Lymphoma, Myeloma Leuk 12:191–196. doi:10.1016/j.clml.2012.01.001
M. R, A. I, I. G, et al. (2009) Activity and safety of lenalidomide and dexamethasone in multiple myeloma patients with advanced renal failure: a Spanish multicenter retrospective study. Blood 114: 749, 2009 (suppl; abstr 1886)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.J. has received honoraria from Janssen, Celgene, Amgen, Gilead, and BMS and has served on the advisory boards of Celgene, Janssen, Roche, Amgen, and BMS. I.C. has received honoraria from Janssen and Gilead. M.N. has received honoraria from Janssen and Celgene and serves on the advisory board of Celgene. P.L. has received honoraria from and serves on the advisory boards of Janssen, Celgene, Amgen, and BMS. C.G. has received honoraria from and serves on the advisory boards of Janssen, Celgene, Amgen, and BMS. F.G. has received honoraria from Celgene. S.E. and J.F. declare no conflict of interests. G.E. has received honoraria from and serves on the advisory boards of Janssen, Celgene, Amgen, and BMS.
Rights and permissions
About this article
Cite this article
João, C., Freitas, J., Gomes, F. et al. Lenalidomide is effective and safe for the treatment of patients with relapsed multiple myeloma and very severe renal impairment. Ann Hematol 95, 931–936 (2016). https://doi.org/10.1007/s00277-016-2662-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2662-6