Abstract
Prolonged isolated thrombocytopenia is a common complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT), which is associated with a poor prognosis. This study aimed to investigate the pathogenesis of prolonged isolated thrombocytopenia (PT). We analysed the expression of CX3CR1 on CD4 and CD8 T cells in bone marrow (BM) and peripheral blood (PB) at +90 days from allo-HSCT recipients with or without PT by flow cytometry analyses. We then determined the megakaryocytes ploidy distributions, apoptosis rate and Fas expression of recipients with or without PT in vitro directly or after depleting CD8+ T cells or adding purified autologous CD8+ T cells to CD8+ T-dep MNCs. We found that the percentage of CD8+ T cells in BM was higher in the patients with PT than in the controls. The elevated expression of the CX3CR1 was associated with PT. There was a marked increase in the percentage of low ploidy megakaryocytes in the recipients with PT. The depletion of CD8+ T cells increased the apoptosis of megakaryocytes and decreased the expression of Fas, which could be corrected by re-adding purified autologous CD8+ T cells. The increase of CD8+ T cells and CD8+/CX3CR1+ T cells in BM at +90 days were independent risk factors for PT according to multivariate analysis. Our data implied that the recruitment of CD8+ T cells into BM might explain the suppression of megakaryocyte apoptosis through the elevated expression of CX3CR1+ in PT after allo-HSCT. CX3CR1 might be a novel treatment target in recipients with PT.





Similar content being viewed by others
References
Zhang X et al (2011) Prolonged thrombocytopenia following allogeneic hematopoietic stem cell transplantation and its association with a reduction in ploidy and an immaturation of megakaryocytes. Biol Blood Marrow Transplant 17(2):274–280
Yamazaki R et al (2006) Prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation: associations with impaired platelet production and increased platelet turnover. Bone Marrow Transplant 38(5):377–384
Kong Y et al (2014) Association between an impaired bone marrow vascular microenvironment and prolonged isolated thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 20(8):1190–1197
Kim DH et al (2006) Prognostic significance of platelet recovery pattern after allogeneic HLA-identical sibling transplantation and its association with severe acute GVHD. Bone Marrow Transplant 37(1):101–108
Poon LM et al (2013) Romiplostim for delayed platelet recovery and secondary thrombocytopenia following allogeneic stem cell transplantation. Am J Blood Res 3(3):260–264
Shono Y et al (2014) Bone marrow graft-versus-host disease: evaluation of its clinical impact on disrupted hematopoiesis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 20(4):495–500
Pihusch R et al (2002) Hemostatic complications in bone marrow transplantation: a retrospective analysis of 447 patients. Transplantation 74(9):1303–1309
Nash RA et al (1996) The problem of thrombocytopenia after hematopoietic stem cell transplantation. Stem Cells 14(Suppl 1):261–273
Nakamae H et al (2011) Cytopenias after day 28 in allogeneic hematopoietic cell transplantation: impact of recipient/donor factors, transplant conditions and myelotoxic drugs. Haematologica 96(12):1838–1845
Ronghe MD et al (2002) The impact of transfusion of leucodepleted platelet concentrates on cytomegalovirus disease after allogeneic stem cell transplantation. Br J Haematol 118(4):1124–1127
Bruno B et al (2001) Secondary failure of platelet recovery after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 7(3):154–162
Reid R et al (2012) Use of eltrombopag, a thrombopoietin receptor agonist, in post-transplantation thrombocytopenia. Am J Hematol 87(7):743–745
Mattia G et al (2002) Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood 99(3):888–897
Patel SR, Hartwig JH, Italiano JE Jr (2005) The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest 115(12):3348–3354
De Botton S et al (2002) Platelet formation is the consequence of caspase activation within megakaryocytes. Blood 100(4):1310–1317
Li S et al (2007) CD8+ T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br J Haematol 139(4):605–611
Houwerzijl EJ et al (2006) Megakaryocytic dysfunction in myelodysplastic syndromes and idiopathic thrombocytopenic purpura is in part due to different forms of cell death. Leukemia 20(11):1937–1942
Olsson B et al (2008) Recruitment of T cells into bone marrow of ITP patients possibly due to elevated expression of VLA-4 and CX3CR1. Blood 112(4):1078–1084
Brissot E et al (2015) Involvement of the CX3CL1 (fractalkine)/CX3CR1 pathway in the pathogenesis of acute graft-versus-host disease. J Leukoc Biol 97(2):227–235
Zhang Y et al (2005) Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol 174(5):3051–3058
Imai T et al (1997) Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91(4):521–530
Wang Y et al (2013) Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 119(5):978–985
Chang YJ, Huang XJ (2014) Haploidentical SCT: the mechanisms underlying the crossing of HLA barriers. Bone Marrow Transplant 49(7):873–879
Tomer A (2004) Human marrow megakaryocyte differentiation: multiparameter correlative analysis identifies von Willebrand factor as a sensitive and distinctive marker for early (2N and 4N) megakaryocytes. Blood 104(9):2722–2727
Mick R, Williams SF, Bitran JD (1990) Patients at increased risk for late engraftment after transplantation: a novel method for their identification. Bone Marrow Transplant 6(3):185–191
Anasetti C et al (1989) Graft-v-host disease is associated with autoimmune-like thrombocytopenia. Blood 73(4):1054–1058
Sivakumaran M et al (1995) Thrombocytopenia following autologous bone marrow transplantation: evidence for autoimmune aetiology and B cell clonal involvement. Bone Marrow Transplant 15(4):531–536
Kim DH et al (2006) Clinical significance of platelet count at day +60 after allogeneic peripheral blood stem cell transplantation. J Korean Med Sci 21(1):46–51
Bolwell B et al (2004) Prognostic importance of the platelet count 100 days post allogeneic bone marrow transplant. Bone Marrow Transplant 33(4):419–423
Umehara H et al (2004) Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol 24(1):34–40
Yoneda O et al (2000) Fractalkine-mediated endothelial cell injury by NK cells. J Immunol 164(8):4055–4062
Di Rosa F, Pabst R (2005) The bone marrow: a nest for migratory memory T cells. Trends Immunol 26(7):360–366
Tomer A et al (1989) Flow cytometric analysis of megakaryocytes from patients with abnormal platelet counts. Blood 74(2):594–601
Falcieri E et al (2000) Ultrastructural characterization of maturation, platelet release, and senescence of human cultured megakaryocytes. Anat Rec 258(1):90–99
White MJ, Kile BT (2010) Apoptotic processes in megakaryocytes and platelets. Semin Hematol 47(3):227–234
Spencer A, Jackson J, Baulch-Brown C (2001) Enumeration of bone marrow ‘homing’ haemopoietic stem cells from G-CSF-mobilised normal donors and influence on engraftment following allogeneic transplantation. Bone Marrow Transplant 28(11):1019–1022
Italiano JE Jr, Shivdasani RA (2003) Megakaryocytes and beyond: the birth of platelets. J Thromb Haemost 1(6):1174–1182
Geddis AE (2009) The regulation of proplatelet production. Haematologica 94(6):756–759
Acknowledgments
The authors thank Drs. Ming Hou and Jun Peng at the Haematological Oncology Centre at Qilu Hospital of Shandong University in China for their excellent technical support and Dr. X. Long Zheng at the Department of Pathology and Laboratory Medicine at The Children’s Hospital of Philadelphia and The University of Pennsylvania for his critical reading of the manuscript.
This work was supported by The National Key Technology Support Program (No. 2012BAI38B03), National Natural Science Foundation of China (No. 81270643 and No. 81470343) and Seeding Grant for Medicine and Engineering Sciences of Peking University (No. BMU20140389). This work was also supported in part by Beijing Natural Science Foundation (No. 7132194) and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20120001110026). All these funding are received by Xiao-Hui Zhang. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Authorship
Contribution: XJH conceived of and designed the experiments, reviewed the data analysis and reviewed the manuscript; XHZ helped with the design of the study and wrote the manuscript; GXW performed the laboratory testing, performed the statistical analyses and reviewed the manuscript; YRL, LPX and KYL helped with the design of the study and reviewed the manuscript; HHZ, WH, HC, YHC, FR W, JZ W, YC and Y W contributed the patient information and reviewed the manuscript; RF, HXF, MW, ML, YZ and TZ participated in collecting and analysing the data.
Conflict of interest
The authors report no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao-Hui Zhang and Guo-Xiang Wang share the first authorship.
Xiao-Hui Zhang and Xiao-Jun Huang share the co-corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
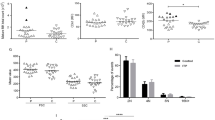
Flow cytometric analysis of lymphocytes from PT or controls. Flow cytometric analysis of lymphocytes from the BM and PB of the recipients with PT (n = 89) or controls (n = 94). CD3+ T cells in the PB (A) of the recipients with PT or controls; CD19+ B cells in the BM (B) and PB (C) of recipients with PT or controls. The results are presented as a percentage and expressed as the mean ± SD. NS, not significant (DOC 229 kb)
Supplementary Fig. 2
Flow cytometric analysis of T lymphocytes from PB of PT or controls. Flow cytometric analysis of T lymphocytes from the PB of the recipients with PT (n = 89) or the controls (n = 94). The results are presented as a percentage and expressed as the mean ± SD. NS, not significant (DOC 157 kb)
Rights and permissions
About this article
Cite this article
Zhang, XH., Wang, GX., Zhu, HH. et al. Recruitment of CD8+ T cells into bone marrow might explain the suppression of megakaryocyte apoptosis through high expression of CX3CR1+ in prolonged isolated thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Ann Hematol 94, 1689–1698 (2015). https://doi.org/10.1007/s00277-015-2436-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2436-6