Abstract
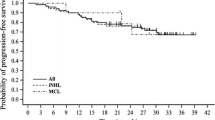
The efficacy/tolerability of bendamustine, a unique alkylator, plus ofatumumab, a human anti-CD20 monoclonal antibody, was evaluated for previously untreated indolent B cell non-Hodgkin’s lymphoma (NHL). The study investigated whether the overall response rate (ORR) for bendamustine-ofatumumab was similar to historical bendamustine-rituximab ORRs (≥90 %). In this multicenter, open-label, single-arm, phase II study, patients received six planned 28-day cycles of bendamustine (90 mg/m2 on days 1 and 2 of each cycle) and ofatumumab (300 mg on day 1, 1000 mg on day 8 of cycle 1, and on day 1 of subsequent cycles). The primary outcome was ORR. Secondary objectives included safety and tolerability. Exploratory evaluations included percentage of patients with positive baseline [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) scans who converted to negative postbaseline and quality of life (QOL) scores. The treated/safety analysis population received ≥1 dose of either therapy. The bendamustine-ofatumumab ORR was 90 % (95 % confidence interval, 77.8–96.6) in 49 treated patients (67 % complete response, 22 % partial response). No patients had progressive disease. Bendamustine-ofatumumab was acceptably tolerated. All 49 patients had ≥1 adverse event, the most common being nausea (61 %), fatigue (55 %), and infusion-related reactions (45 %, all but 1 occurring during cycle 1). The proportion of patients whose FDG-PET scans converted to negative postbaseline was 88 %. Changes in QOL scores were minor. In patients with treatment-naive, indolent B cell NHL, bendamustine-ofatumumab exhibited a high degree of activity (90 % ORR), comparable with historical bendamustine-rituximab ORRs (≥90 %), and was adequately tolerated (ClinicalTrials.gov identifier: NCT01108341).

Similar content being viewed by others
References
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Dürk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W, Study group indolent Lymphomas (StiL) (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381:1203–1210. doi:10.1016/S0140-6736(12)61763-2
Czuczman MS, Grillo-López AJ, White CA, Saleh M, Gordon L, LoBuglio AF, Jonas C, Klippenstein D, Dallaire B, Varns C (1999) Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 17:268–276
Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, Solal-Celigny P, Offner F, Walewski J, Raposo J, Jack A, Smith P (2005) CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 105:1417–1423
Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L, Burke JM (2014) Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 123:2944–2952. doi:10.1182/blood-2013-11-531327
Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, Elliott G, Niemeyer CC (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 14:309–317. doi:10.1158/1078-0432.CCR-07-1061
Tageja N (2011) Bendamustine: safety and efficacy in the management of indolent non-Hodgkin’s lymphoma. Clin Med Insights Oncol 5:145–156. doi:10.4137/CMO.S6085
Czuczman MS, Hess G, Gadeberg OV, Pedersen LM, Goldstein N, Gupta I, Jewell RC, Lin TS, Lisby S, Strange C, Windfeld K, Viardot A, 409 Study Investigators (2012) Chemoimmunotherapy with ofatumumab in combination with CHOP in previously untreated follicular lymphoma. Br J Haematol 157:438–445. doi:10.1111/j.1365-2141.2012.09086.×
Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, Link BK, Robak T, Wojtukiewicz M, Pfreundschuh M, Kneba M, Engert A, Sonneveld P, Flensburg M, Petersen J, Losic N, Radford J (2008) First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood 111:5486–5495. doi:10.1182/blood-2007-10-117671
Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H, McDougal A, Pilaro A, Chiang R, Gootenberg JE, Keegan P, Pazdur R (2010) U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res 16:4331–4338. doi:10.1158/1078-0432.CCR-10-0570
Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C, Parren PW, Hack CE, Dechant M, Valerius T, van de Winkel JG, Glennie MJ (2004) Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104:1793–1800
Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, van Meerten T, Ebeling S, Vink T, Slootstra JW, Parren PW, Glennie MJ, van de Winkel JG (2006) The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 177:362–371
Czuczman MS, Fayad L, Delwail V, Cartron G, Jacobsen E, Kuliczkowski K, Link BK, Pinter-Brown L, Radford J, Hellmann A, Gallop-Evans E, DiRienzo CG, Goldstein N, Gupta I, Jewell RC, Lin TS, Lisby S, Schultz M, Russell CA, Hagenbeek A, 405 Study Investigators (2012) Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood 119:3698–3704. doi:10.1182/blood-2011-09-378323
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
Trotman J, Fournier M, Lamy T, Seymour JF, Sonet A, Janikova A, Shpilberg O, Gyan E, Tilly H, Estell J, Forsyth C, Decaudin D, Fabiani B, Gabarre J, Salles B, Van Den Neste E, Canioni D, Garin E, Fulham M, Vander Borght T, Salles G (2011) Positron emission tomography–computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol 29:3194–3200. doi:10.1200/JCO.2011.35.0736
Acknowledgments
This research was sponsored and conducted by Teva Branded Pharmaceutical Products R&D, Inc., Frazer, PA. Ofatumumab was provided by GlaxoSmithKline, Brentford, UK. Special thanks are also given to all patients and their families, all participating sites, and research staff for their dedication to this study (see Appendix for listing of contributing investigators and participating sites). The authors thank Peter Brown (Teva) for his gracious review of this manuscript. Funding for editorial, design, and production support was provided by Teva to The Curry Rockefeller Group, LLC, Tarrytown, NY.
Conflict of interest
Myron S. Czuczman is a consultant to GlaxoSmithKline and Teva Branded Pharmaceutical Products R&D, Inc., has received honoraria from Teva, and has received research funding from Cephalon (now Teva). Andres Forero’s institution has received research funding from Cephalon (now Teva). Glen Davis is an employee of Teva. Mihaela Munteanu is an employee and shareholder of Teva and has owned stock/held an ownership interest in Janssen. Fritz Offner is a consultant to GlaxoSmithKline. Nathan Fowler is a consultant to Roche and has received research funding from Roche and Teva. Stephen Kahanic, Eric Van Den Neste, Dominique Bron, and Donald Quick declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Change from baseline to last observation in European Organization for Research and Treatment of Cancer 30-item core quality of life (QOL) Questionnaire scores: (a). Increases in g lobal health status/QOL and function scale scores indicate increased function (improvement) (b). decreases in symptom scales indicate decreased symptoms (improvement). (DOC 104 kb)
Appendix
The following investigators contributed to this study:
Belgium—Dominique Bron, M.D., Ph.D. (Institut Jules Bordet, Brussels); Fritz Offner, M.D. (Universitair Ziekenhuis Gent, Gent); Eric Van Den Neste, M.D. (Cliniques Universitaires Saint-Luc, Brussels); USA—Arvind Aggarwal, M.D. (Dublin Hematology Oncology Care, Dublin, GA); Thomas Boyd, M.D. (Yakima Valley Memorial Hospital/North Start Lodge, Yakima, WA); Myron Czuczman, M.D. (Roswell Park Cancer Institute, Buffalo, NY); Delva Deauna-Limayo, M.D. (Nevada Cancer Institute, Las Vegas, NV); William Edenfield, M.D. (Cancer Centers of the Carolinas, Greenville, SC); Andres Forero, M.D. (University of Alabama at Birmingham, Birmingham, AL); Nathan Fowler, M.D. (The University of Texas MD Anderson Cancer Center, Houston, TX); Hassan Ghazal, M.D. (Kentucky Cancer Clinic, Hazard, KY); Robert Hermann, M.D., FACP (Northwest Georgia Oncology Center, Marietta, GA); Samuel Jacobs, M.D. (University of Pittsburgh Medical Center, Pittsburgh, PA); Stephen Kahanic, M.D. (Siouxland Hematology-Oncology Associates, Sioux City, IA); Dean Kirkel, M.D. (University Cancer Institute, Boynton Beach, FL); Dhatri Kodali, M.D. (Texas Oncology, Webster, TX); Jonathan Kolitz, M.D., FACP (Monter Cancer Center, Lake Success, NY); Shailendra Lakhanpal, M.D. (Birmingham Hematology and Oncology Associates, Birmingham, AL); Craig Okada, M.D. (Oregon Health Sciences University, Portland, OR); Dipti Patel-Donnelly, M.D. (Fairfax Northern Virginia Hematology Oncology, Fairfax, VA); Donald Quick, M.D. (Joe Arrington Cancer Research and Treatment Center, Lubbock, TX); Mansoor Saleh, M.D. (Georgia Cancer Specialists, Tucker, GA); Bradley Somer, M.D. (The West Clinic, Memphis, TN).
Rights and permissions
About this article
Cite this article
Czuczman, M.S., Kahanic, S., Forero, A. et al. Results of a phase II study of bendamustine and ofatumumab in untreated indolent B cell non-Hodgkin’s lymphoma. Ann Hematol 94, 633–641 (2015). https://doi.org/10.1007/s00277-014-2269-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2269-8