Abstract
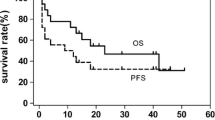
The prognosis of extranodal nature killer (NK)/T cell lymphoma (ENKL) is dismal because of its aggressive course and multidrug resistance. Currently, for patients with relapsed/refractory ENKL, l-asparaginase-based regimens such as l-asparaginase, ifosfamide, methotrexate, etoposide, and dexamethasone (SMILE) or l-asparaginase, methotrexate, and dexamethasone (AspaMetDex) are recommended. We retrospectively investigated the efficacy and safety of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of 17 relapsed/refractory ENKL patients. Clinical data from these patients were collected and analyzed. The primary end point was overall response rate (ORR). All patients were subjected to 2 to 6 cycles of DDGP chemotherapy, and the median number of cycles of DDGP regimen administrated was four. The ORR was 88.2 % (15/17), with nine patients (52.9 %) achieved complete response (CR) and six patients (35.3 %) achieved partial response (PR). The median follow-up time was 17 months (range 2–28 months). The 1-year overall survival (OS) rate and 1-year progression-free survival (PFS) were 82.4 and 64.7 %, respectively. For those CR responders, the median PFS was 17 months. Grade 3/4 neutropenia occurred in nine patients (52.9 %) and grade 3/4 thrombocytopenia occurred in six patients (35.3 %). DDGP combination chemotherapy produces favorable outcomes in relapsed/refractory ENKL, and more attention should be paid to treatment-related myelosuppression. Further prospective trials are expected to define the efficacy.


Similar content being viewed by others
References
Au WY, Weisenburger DD, Intragumtornchai T et al (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113:3931–3937
Tse E, Kwong YL (2013) How I treat NK/T-cell lymphomas. Blood 121:4997–5005
Ito Y, Kimura H, Maeda Y et al (2012) Pretreatment EBV-DNA copy number is predictive of response and toxicities to SMILE chemotherapy for extranodal NK/T-cell lymphoma, nasal type. Clin Cancer Res 18:4183–4190
Kwong YL, Anderson BO, Advani R et al (2009) Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 10:1093–1101
Kim SJ, Kim K, Kim BS et al (2009) Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol 27:6027–6032
Yamaguchi M, Kita K, Miwa H et al (1995) Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 76:2351–2356
Tse E, Kwong YL (2012) Practical management of natural killer/T-cell lymphoma. Curr Opin Oncol 24:480–486
Yamaguchi M, Kwong YL, Kim WS et al (2011) Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell tumor study group study. J Clin Oncol 29:4410–4416
Jaccard A, Gachard N, Marin B et al (2011) Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117:1834–1839
Tsukune Y, Isobe Y, Yasuda H et al (2010) Activity and safety of combination chemotherapy with methotrexate, ifosfamide, l-asparaginase and dexamethasone (MILD) for refractory lymphoid malignancies: a pilot study. Eur J Haematol 84:310–315
Morschhauser F, Depil S, Jourdan E et al (2007) Phase II study of gemcitabine-dexamethasone with or without cisplatin in relapsed or refractory mantle cell lymphoma. Ann Oncol 18:370–375
Dong M, He XH, Liu P et al (2013) Gemcitabine-based combination regimen in patients with peripheral T-cell lymphoma. Med Oncol 30:351
Mahadevan D, Unger JM, Spier CM et al (2013) Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer 119:371–379
Zinzani PL, Venturini F, Stefoni V et al (2010) Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol 21:860–863
Escherich G, Zur Stadt U, Zimmermann M et al (2013) Clofarabine in combination with pegylated asparaginase in the frontline treatment of childhood acute lymphoblastic leukaemia: a feasibility report from the CoALL 08-09 trial. Br J Haematol 163:240–247
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Ando M, Sugimoto K, Kitoh T et al (2005) Selective apoptosis of natural killer-cell tumours by L-asparaginase. Br J Haematol 130:860–868
Charamella LJ, Meyer C, Thompson GE et al (1985) Chemotherapeutic agents and modulation of natural killer cell activity in vitro. J Immunopharmacol 7:53–65
Jaccard A, Petit B, Girault S et al (2009) L-asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol 20:110–116
Yong W, Zheng W, Zhu J et al (2009) L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 88:647–652
Li L, Zhang C, Zhang L et al (2014) Efficacy of apegaspargase-based regimen in the treatment of newly-diagnosed extranodal natural killer/T-cell lymphoma. Neoplasma 61:225–232
Reyes VE Jr, Al-Saleem T, Robu VG et al (2010) Extranodal NK/T-cell lymphoma nasal type: efficacy of pegaspargase. Report of two patients from the United Sates and review of literature. Leuk Res 34:e50–e54
Evens AM, Rosen ST, Helenowski I et al (2013) A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br J Haematol 163:55–61
Mounier N, El Gnaoui T, Tilly H et al (2013) Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica 98:1726–1731
Ahn HK, Kim SJ, Hwang DW et al (2013) Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Investig New Drugs 31:469–472
Kim TM, Kim S, Ahn YO et al (2014) Anti-cancer activity of gemcitabine against natural killer cell leukemia/lymphoma. Leuk Lymphoma 55:940–943
Dinndorf PA, Gootenberg J, Cohen MH et al (2007) FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist 12:991–998
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant number 81172118].
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Li, X., Chen, C. et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol 93, 1889–1894 (2014). https://doi.org/10.1007/s00277-014-2136-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2136-7