Abstract
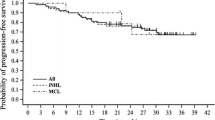
Bendamustine is an alkylating agent with a nitrogen mustard group and a purine-like benzimidazole group. The aim of this study was to collect all the Italian experiences with this drug in order to evaluate the results in term of response to therapy and toxicities. We analyzed lymphoma patients treated in 24 Italian haematological centres with bendamustine alone or in combination with anti-CD20 antibody. One hundred seventy-five relapsed or refractory lymphoma patients were enrolled. The median age was 69 years (range 26–87). Seventy-nine patients were relapsed, 35 were refractory and 61 presented a progressive disease after partial response. The diagnoses were 60 indolent non-follicular lymphomas, 34 diffuse large B-cell lymphomas, 48 follicular lymphomas, 30 mantle cell lymphomas and three peripheral T-cell lymphomas. All patients were evaluable for response: 52 (29%) with complete remission, 72 (43%) with partial response with an overall response rate of 71%, and 51 non-responders. With a median observation period of 10 months (1–43), 70% of patients are alive. In summary, this retrospective study shows that treatment with bendamustine alone or in combination with rituximab is a safe and effective regimen in a subset of multi-resistant patients.






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics 2008. CA Cancer J Clin 58:71–96
Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. Ann Oncol 16:481–488
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelik HK, Vardiman J, Lister TA, Bloomfield CD (2000) The Word Health Organization Classification of hematological malignancies report of the clinical advisory committee meeting, Airlie House, Virginia, November 1997. Mod Pathol 13(2):193–207
Vose JM, Bierman PJ, Anderson JR, Kessinger A, Pierson J, Nelson J, Prappier B, Schmit-Pokorny K, Weisenburger DD, Armitage JO (1992) Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood 80(8):2142–2148
Elstrom RL, Martin P, Ostrow K, Barrientos J, Chadburn A, Furman R, Ruan J, Shore T, Schuster M, Cerchietti L, Melnick A, Coleman M, Leonard JP (2010) Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk 10(3):192–196
Barman Balfour JA, Goa KL (2001) Bendamustine. Drugs 61(5):631–638
Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, Elliot G, Niemeyer CC (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkilating agents. Clin Cancer Res 14:309–317
Strumberg D, Harsdtrick A, Doll K, Hoffmann B, Seeber S (1996) Bendamustine hydrochloride activity against doxorubicin-resistent human breast carcinoma cell lines. Anticancer Drugs 7:415–421
Leoni LM, Bailey B, Reifert J (2003) SDX-105 (bendamustine), a clinically active antineoplastic agent posesses a unique mechanism of action. Blood; 102:640a
Montillo M, Ricci F, Tedeschi A, Vismara E, Morra E (2010) Bendamustine: new perspective for an old drug in lymphoprolipherative disorders. Expert Rev Hematol 3(2):131–148
Friedberg JW, Cohen P, Chen L, Robinson KS, Forero-Torres A, La Casce AS, Fayad LE, Bessudo A, Camacho ES, Williams ME, van der Jagt RH, Oliver JW, Cheson BD (2008) Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin's lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol 26:204–210
Bremer K (2002) High rates of long-lasting remissions after 5-day bendamustine chemotherapy cycles in pre-treated low-grade non-Hodgkin's lymphoma. J Cancer Res Clin Oncol 128:603–609
Ujjani C, Cheson B (2011) Efficacy of bendamustine in rituximab-refractory indolent B-cell non-Hodgkin lymphoma: review of a pivotal trial. Future Oncol 7(1):9–14
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol 17:1244–1253
SPSS Inc. (1998) SPSS for Windows, version 12.0. Chicago: SPSS.
Heider A, Niederle N (2001) Efficacy and toxicity of bendamustine in patients with relapsed low-grade non-Hodgkin's lymphoma. Anticancer Drugs 12:725–729
Kath R, Blumenstengel K, Fricke HJ, Hoffken K (2001) Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia. J Cancer Research Clin Oncol 127:48–54
Friedberg JW, Vose JM, Kelly JL, Young F, Bernstein SH, Peterson D, Rich L, Blumel S, Proia NK, Liesveld J, Fisher RI, Armitage JO, Grant S, Leonard JP.(2011) The combination of bendamustine,bortezomib and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood, Jan 14 Epub
Rummel MJ, Al-Batran SE, Kim SZ, Welsalu M, Hecker R, Kofahl-Krause D, Josten KM, Durk H, Rost A, Neise M, von Grunhagen U, Chow KU, Hansmann ML, Holzer D, Mitrou PS (2005) Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low grade non-Hodgkin’s lymphoma. J Clin Oncol 23(15):3383–3389
Cheson BD, Friedberg JW, Kahl BS, Van der Jagt RH, Tremmel L (2010) Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 10(6):452–457
Ohmachi K, Ando K, Ogura M, Uchida T, Itoh K, Kubota N, Ishizawa K, Yamamoto J, Watanabe T, Uike N, Choi I, Terui Y, Usuki K, Nagai H, Uoshima N, Tobinai K (2010) Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci 101(9):2059–2064
Weide R, Pandorf A, Heymanns J et al (2004) Bendamustine/mitoxantrone/rituximab (BMR): a very effective, well tolerated outpatient chemoimmunotherapy for relapsed and refractory CD20 positive indolent malignancies. Final results of a pilot study Leukemia and Lymphoma 45:2445–2449
Lissitchkov T, Arnaudov G, Peytchev D, Merkle KH (2006) Phase I/II study to evaluate dose limiting toxicity, massimum tolerated doses, and tolerability of bendamustine HCL in pre-treated patients with B-chronic lymphocytic leukemia (binet stage) requiring therapy. J Cancer Res Clin Oncol 132:99–104
Schoffski P, Seeland G, Engel H, Grunwald V, Paul H, Merkle K, Kowalski R, Ganser A (2000) Weekly administration of bendamustine a phase I study in patients with advanced progressive solid tumors. Ann Oncol 11:729–735
Rasschaert M, Schrijvers D, Van den Brande J, Dyck J, Bosmans J, Merkle K, Vermorken JB (2007) A phase I study of bendamustine hydrochloride administered day 1 + 2 every 3 weeks in patients with solid tumors. Br J Cancer 96:1692–1698
Cheson BD, Wendtner CM, Pieper A, Dreyling M, Friedberg J, Hoelzer D, Moreau P, Gribben J, Knop S, Montillo M, Rummel M (2010) Optimal use of bendamustine in chronic lymphocytic leukemia, non Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk 10:21–27
Rummel MJ, Niederle N, Maschmeyer G, Banat A, von Gruenhagen U, Losem C, Heil G, Welslau M, Balser C, Kaiser U, Ballo H, Weidmann E, Duerk HA, Kofahl-Krause D, Roller F, Barth J, Hoelzer D, Hinke A and Brugger W. (2009) Bendamustine plus rituximab is superior in respect of progression free survival and CR rate when compared to CHOP plus rituximab as first-line treatment of patients with advanced follicular, indolent, and mantle cell lymphomas: final results of a randomized phase III study of the StiL (Study Group Indolent Lymphomas, Germany). Abs. 405 :51st ASH Meeting
Acknowledgements
The following researchers have substantially contributed to data acquisition: Maurizio Musso, UO Ematologia e Trapianto di Midollo, CDC "La Maddalena", Palermo; Pellegrino Musto, UO di Ematologia e Trapianto di Cellule Staminali, IRCCS-CROB, Rionero in Vulture; Teodoro Chisesi, UO Ematologia Mestre-Venezia; Nicola Di Renzo, UO Ematologia e Trapianto di cellule Staminali, P.O V. Fazzi, Lecce; Nicola Cascavilla, Ematologia IRCS Casa Sollievo della Sofferenza, San Giovanni Rotondo; Caterina Stelitano, UO Ematologia Ospedali Melacrino-Morelli, Reggio Calabria; and Mariella Sciacca, UO OncoEmatologia AO Sant’Andrea, Vercelli.
Financial disclosures
There are no financial disclosures from any authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rigacci, L., Puccini, B., Cortelazzo, S. et al. Bendamustine with or without rituximab for the treatment of heavily pretreated non-Hodgkin’s lymphoma patients. Ann Hematol 91, 1013–1022 (2012). https://doi.org/10.1007/s00277-012-1422-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1422-5