Abstract
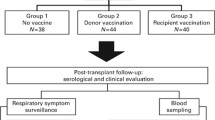
Vaccination is the best strategy to prevent influenza infection that is a potential cause of morbidity and mortality in immunosuppressed patients. Here, we evaluated the factors that may affect serological response to influenza vaccine in patients who have undergone hematopoetic stem cell transplantation (HSCT). Sixty-one HSCT recipients were included in the study during the 2007–2008 influenza season. Serum samples prior to vaccination and 6–10 weeks after vaccination were collected. Samples were assayed for antibodies to influenza virus A/H1N1, A/H3N2, and B strains by hemagglutination-inhibition assay. The patients were followed in terms of clinical symptoms up to the next influenza season and for adverse effects within a month after vaccination. Overall, pre-vaccine seroprotection rate against all vaccine antigens (A/H1N1, A/H3N2, and B antigens) was 45.1%, post-vaccine seroprotection rate 91% and seroconversion rate was 28.3%. Seroconversion rates were found to be low against B in patients who were vaccinated in the late influenza season (p = 0.018; respectively). Five patients (10.9%) had no immune response against H1N1. Adverse events were reported in 19.6% (n = 9/46) of the patients. In conclusion, the patients should be vaccinated as early as possible in the influenza season, before they are exposed to the virus.
Similar content being viewed by others
References
Ljungman P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ et al (2001) Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 28:479–484
Ljungman P, Avetisyan G (2008) Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 42:637–641
Machado CM, Cardoso MRA, da Rocha IF, Boas LSV, Dulley FL (2005) The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant 36:897–900
Raboni SM, Nogueira MB, Tsuchiya LR, Takahashi GA, Pereira LA, Pasquini R, Siqueira MM (2003) Respiratory tract viral infections in bone marrow transplant patients. Transplantation 76:142–146
Nichols WG, Guthrie KA, Corey L, Boeckh M (2004) Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 39:1300–1306
Whimbey E, Elting LS, Couch RB, Lo W, Williams L, Champlin RE, Bodey GP (1994) Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant 13:437–440
Bowden RA (1997) Respiratory virus infections after marrow transplant: The Fred Hutchinson Cancer Research Center Experience. Am J Med 102:27–30
Cough RB, Englund JA, Whimbley E (1997) Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med 102:2–9
Scott JD, Englund JA, Myerson D, Geballe AP (2004) Influenza a pneumonia presenting as progressive focal infiltrates in a stem cell transplant recipient. J Clin Virol 31:96–99
Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G et al (2008) Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) 2008. MMWR Recomm Rep 57:1–60
Van Essen GA, Palache AM, Forleo E, Fedson DS (2003) Influenza vaccination in 2000: recommendations and vaccine use in 50 developed and rapidly developing countries. Vaccine 21:1780–1785
Ljungman P, Engelhard D, de la Camara R, Einsele H, Locasciulli A, Martino R et al (2005) Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant 35:737–746
Machado CM (2004) Reimmunization after bone marrow transplantation-current recommendations and perspectives. Braz J Med Biol Res 37:151–158
Zimmerman RK, Middleton DB (2007) Vaccines for persons at high risk. J Fam Pract 56(2):S38–S46
Wilck MB, Baden LR (2008) Vaccination after stem cell transplant: a review of recent developments and implications for current practice. Curr Opin Infect Dis 21:399–408
Avetisyan G, Aschan J, Hassan M, Ljungman P (2008) Evaluation of immune responses to seasonal influenza vaccination in healthy volunteers and in patients after stem cell transplantation. Transplantation 86:257–263
Pauksen K, Linde A, Hammarström V, Sjölin J, Carneskog J, Jonsson G, Oberga G, Engelmann H, Ljungman P (2000) Granulocyte-macrophage colony-stimulating factor as immunomodulating factor together with influenza vaccination in stem cell transplant patients. Clin Infect Dis 30:342–348, Erratum in: Clin Infect Dis 2000; 30: 992
Morecki S, Gelfand Y, Nagler A, Or R, Naparstek E, Varadi G, Engelhard D, Akerstein A, Slavin S (2001) Immune reconstitution following allogeneic stem cell transplantation in recipients conditioned by low intensity vs myeloablative regimen. Bone Marrow Transplant 28:243–249
Ring A, Marx G, Steer C, Harper P (2002) Influenza vaccination and chemotherapy: a shot in the dark? Support Care Cancer 10:462–465
Engelhard D, Nagler A, Hardan I, Morag A, Aker M, Baciu H et al (1993) Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell depleted and autologous BMT recipients. Bone Marrow Transplant 11:1–5
Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, Sandmaier B, Maloney D, Storb R, Storek J (2003) Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol 31:941–952
Gandhi MK, Egner W, Sizer L, Inman I, Zambon M, Craig JI, Marcus RE (2001) Antibody responses to vaccinations given within the first two years after transplant are similar between autologous peripheral blood stem cell and bone marrow transplant recipients. Bone Marrow Transplant 28:775–781
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yalçın, S.S., Kondolot, M., Albayrak, N. et al. Serological response to influenza vaccine after hematopoetic stem cell transplantation. Ann Hematol 89, 913–918 (2010). https://doi.org/10.1007/s00277-009-0897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-009-0897-1