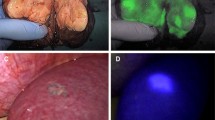
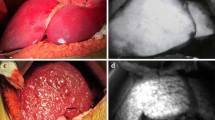
Abstract
Purpose
To evaluate an integrated liver biopsy platform that combined CT image fusion, electromagnetic (EM) tracking, and optical molecular imaging (OMI) of indocyanine green (ICG) to target hepatocellular carcinoma (HCC) lesions and a point-of-care (POC) OMI to assess biopsy cores, all based on tumor retention of ICG compared to normal liver, in phantom and animal model.
Material
A custom CT image fusion and EM-tracked guidance platform was modified to integrate the measurement of ICG fluorescence intensity signals in targeted liver tissue with an OMI stylet or a POC OMI system. Accuracy was evaluated in phantom and a woodchuck with HCC, 1 day after administration of ICG. Fresh biopsy cores and paraffin-embedded formalin-fixed liver tissue blocks were evaluated with the OMI stylet or POC system to identify ICG fluorescence signal and ICG peak intensity.
Results
The mean distance between the initial guided needle delivery location and the peak ICG signal was 5.0 ± 4.7 mm in the phantom. There was complete agreement between the reviewers of the POC-acquired ICG images, cytology, and histopathology in differentiating HCC-positive from HCC-negative biopsy cores. The peak ICG fluorescence intensity signal in the ex vivo liver blocks was 39 ± 12 and 281 ± 150 for HCC negative and HCC positive, respectively.
Conclusion
Biopsy guidance with fused CT imaging, EM tracking, and ICG tracking with an OMI stylet to detect HCC is feasible. Immediate assessment of ICG uptake in biopsy cores with the POC OMI system is feasible and correlates with the presence of HCC in the tissue.

source is entering the light-blocking box by cable. D Integrated interface for co-display of the CT-fused EM-tracked cannula triplanar position and ICG signal read-out of the OMI stylet. E ICG fluorescence intensity while withdrawing the OMI stylet through the ICG injection site with measurements at approximately 5-mm intervals. Gaussian distribution is accurate and was added to demonstrate rough orientation only. Note that the OMI stylet data sampling is directional. Thus, the signal distribution would not be circumferential nor Gaussian with mirrored shoulders, even if the ICG distribution was spherical and uniform



Similar content being viewed by others
References
Abi-Jaoudeh N, Kruecker J, Kadoury S, Kobeiter H, Venkatesan AM, Levy E, et al. Multimodality image fusion-guided procedures: technique, accuracy, and applications. Cardiovasc Interv Radiol. 2012;35(5):986–8.
Krucker J, Xu S, Venkatesan A, Locklin JK, Amalou H, Glossop N, et al. Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol. 2011;22(4):515–24.
Bhattacharji P, Moore W. Application of real-time 3D navigation system in CT-guided percutaneous interventional procedures: a feasibility study. Radiol Res Pract. 2017;2017:3151694.
Sheth RA, Arellano RS, Uppot RN, Samir AE, Goyal L, Zhu AX, et al. Prospective trial with optical molecular imaging for percutaneous interventions in focal hepatic lesions. Radiology. 2015;274(3):917–26.
Sheth RA, Heidari P, Esfahani SA, Wood BJ, Mahmood U. Interventional optical molecular imaging guidance during percutaneous biopsy. Radiology. 2014;271(3):770–7.
Hakime A, Barah A, Deschamps F, Farouil G, Joskin J, Tselikas L, et al. Prospective comparison of freehand and electromagnetic needle tracking for US-guided percutaneous liver biopsy. J Vasc Interv Radiol. 2013;24(11):1682–9.
Appelbaum L, Solbiati L, Sosna J, Nissenbaum Y, Greenbaum N, Goldberg SN. Evaluation of an electromagnetic image-fusion navigation system for biopsy of small lesions: assessment of accuracy in an in vivo swine model. Acad Radiol. 2013;20(2):209–17.
Kim E, Ward TJ, Patel RS, Fischman AM, Nowakowski S, Lookstein RA. CT-guided liver biopsy with electromagnetic tracking: results from a single-center prospective randomized controlled trial. AJR Am J Roentgenol. 2014;203(6):W715–23.
Tinguely P, Frehner L, Lachenmayer A, Banz V, Weber S, Candinas D, et al. Stereotactic image-guided microwave ablation for malignant liver tumors—a multivariable accuracy and efficacy analysis. Front Oncol. 2020;10:842.
Yamamoto S, Matsumoto T, Suda S, Tomita K, Kamei S, Hashida K, et al. First experience of efficacy and radiation exposure in 320-detector row CT fluoroscopy-guided interventions. Br J Radiol. 2021;94(1120):20200754. https://doi.org/10.1259/bjr.20200754.
Schaible J, Lürken LA-OX, Wiggermann P, Verloh NA-O, Einspieler I, Zeman F, et al. Primary efficacy of percutaneous microwave ablation of malignant liver tumors: comparison of stereotactic and conventional manual guidance. Sci Rep. 2020;10(1):1–8.
Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600.
Engel E, Schraml R, Maisch T, Kobuch K, Konig B, Szeimies RM, et al. Light-induced decomposition of indocyanine green. Invest Ophthalmol Vis Sci. 2008;49(5):1777–83.
He P, Huang T, Fang C, Su S, Tian J, Xia X, et al. Identification of extrahepatic metastasis of hepatocellular carcinoma using indocyanine green fluorescence imaging. Photodiagnosis Photodyn Ther. 2019;25:417–20.
Nakaseko Y, Ishizawa T, Saiura A. Fluorescence-guided surgery for liver tumors. J Surg Oncol. 2018;118(2):324–31.
Benedicenti S, Molfino S, Alfano MS, Molteni B, Porsio P, Portolani N, et al. Indocyanine-green fluorescence-GUIDED liver resection of metastasis from squamous cell carcinoma invading the biliary tree. Case Rep Gastrointest Med. 2018;2018:5849816.
Pritchard WF, Woods DL, Esparza-Trujillo JA, Starost MF, Mauda-Havakuk M, Mikhail AS, et al. Transarterial chemoembolization in a woodchuck model of hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(5):812–9. https://doi.org/10.1016/j.jvir.2019.08.031.
Masuda K, Kaneko J, Kawaguchi Y, Togashi J, Arita J, Akamatsu N, et al. Diagnostic accuracy of indocyanine green fluorescence imaging and multidetector row computed tomography for identifying hepatocellular carcinoma with liver explant correlation. Hepatol Res. 2017;47(12):1299–307.
Kaibori M, Matsui K, Ishizaki M, Iida H, Sakaguchi T, Tsuda T, et al. Evaluation of fluorescence imaging with indocyanine green in hepatocellular carcinoma. Cancer Imag. 2016;16:6.
Burke CT, Cullen JM, State A, Gadi S, Wilber K, Rosenthal M, et al. Development of an animal model for radiofrequency ablation of primary, virally induced hepatocellular carcinoma in the woodchuck. J Vasc Interv Radiol. 2011;22(11):1613–8. https://doi.org/10.1016/j.jvir.2011.08.020.
Russo FP, Imondi A, Lynch EN, Farinati F. When and how should we perform a biopsy for HCC in patients with liver cirrhosis in 2018? A review. Dig Liver Dis. 2018;50(7):640–6.
Sparchez Z, Mocan T. Contemporary role of liver biopsy in hepatocellular carcinoma. World J Hepatol. 2018;10(7):452–61.
Liu J, Dang H, Wang XW. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp Mol Med. 2018;50(1):16.
Wang XW, Thorgeirsson SS. The biological and clinical challenge of liver cancer heterogeneity. Hepat Oncol. 2014;1(4):349–53.
Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell. 2019;36(4):418-30.e6.
Lee J-M, Hays JL, Noonan AM, Squires J, Minasian L, Annunziata C, et al. Feasibility and safety of sequential research-related tumor core biopsies in clinical trials. Cancer. 2013;119(7):1357–64.
Yamaguchi T, Kokudo T, Akamatsu N, Kaneko J, Arita J, Sakamoto Y, et al. Liver Regeneration is Preserved After At Least Four Repeated Liver Resections for Hepatocellular Carcinoma. World J Surg. 2018;42(12):4070–80.
Funding
This work was supported by the NIH Center for Interventional Oncology and the Intramural Research Program of the National Institutes of Health, via intramural NIH Grants Z1A CL040015, 1ZID BC011242; also supported by NIH UO1 (NCI Collaborative Grant) # 1U01CA202934-01A1. Dr. Mauda-Havakuk is supported by the Intramural Program of the National Institute of Biomedical Imaging and Bioengineering. NIH has a Materials Transfer Agreement with Northeastern Wildlife.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
NIH may have intellectual property in the field. BW is Principal Investigator on a CRADA (Cooperative Research & Development Agreement) between NIH and Philips and Philips Research. Licensed Patents / Royalties: Philips pays royalties to NIH for a licensing agreement with NIH, who then pays royalties to BW for licensed patents from Philips. UM has co-invented quantitative optical imaging methods and activatable optical imaging agents, for which MGH was issued patents. He is a cofounder, shareholder, consultant, and grant recipient of Cytosite Biopharma, focused on immuno-oncology PET imaging. All other authors declare that they have no conflict of interest: QR, SX, ML, WP, MS, AF, AM, MM, JE, IB, PH, and JK.
Ethical Approval
This article does not contain any studies with human participants. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. FDA (± CE Mark): Off-label use of drugs or devices may be discussed. The content of this manuscript does not necessarily reflect the views, policies, or opinions of the U.S. Department of Health and Human Services. The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as an actual or implied endorsement of such products by the United States Government. Opinions expressed are those of the authors, not necessarily the NIH.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Ruiter, Q.M.B., Xu, S., Li, M. et al. Electromagnetic Tracking and Optical Molecular Imaging Guidance for Liver Biopsy and Point-of-Care Tissue Assessment in Phantom and Woodchuck Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 44, 1439–1447 (2021). https://doi.org/10.1007/s00270-021-02853-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02853-x