Abstract
Purpose
To compare the survival outcomes of patients treated with transarterial ethanol ablation (TEA) with those treated with liver resection (LR) for solitary HCC less than 5 cm in diameter, in patients stratified according to liver function using ALBI grade.
Materials and Methods
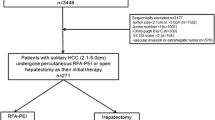
This retrospective study approved by the Institutional Committee included all treatment-naïve patients with solitary HCC (≤ 5 cm) and Child–Pugh score 5, and who had received TEA (33 patients) or LR (192 patients) between 2004 and 2012. Treatment outcomes were compared between patients treated with TEA and LR after a period of at least 7 years of follow-up. Comparison was repeated for those patients with ALBI grade 2 or 3.
Results
Both overall survival (OS, months) and recurrence-free survival (RFS months) were significantly longer in the LR group (OS: LR 129.7[119.5, 140], TEA 69.1[55.9, 82.3], P < 0.0001; RFS: LR 91.3[43.5, 139.1], TEA 13.8 [11, 16.5], P < 0.0001). In patients with ALBI grade 2 or 3, there was no significant difference between the groups in OS or RFS (OS: LR 43.1[0, 91.2], TEA 55.4 [43.7, 67.2], P = 0.65; RFS: LR 17.8 [11.4, 24.2], TEA 11.9 [6.7, 17.1], P = 0.132). Transient epigastric discomfort and low-grade fever without consequence occurred in 8 patients (8/33 or 24.2%) in the TEA group.
Conclusion
The overall survival after LR for HCCs ≤ 5 cm was superior to that after TEA but similar when compared in patients with ALBI grade 2 or 3, the ALBI grade is useful for patient selection for TEA or LR for HCCs ≤ 5 cm.






Similar content being viewed by others
References
Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol. 2017;3:1683–91. https://doi.org/10.1001/jamaoncol.2017.3055.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50. https://doi.org/10.1002/hep.29913.
Yau T, Tang VYF, Yao TJ, Fan ST, Lo CM, Poon RTP. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(1691–1700):e3. https://doi.org/10.1053/j.gastro.2014.02.032.
Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona clinic liver cancer stages: a multicentre study. J Hepatol. 2015;62:617–24. https://doi.org/10.1016/j.jhep.2014.10.037.
Qi X, Zhao Y, Li H, Guo X, Han G. Management of hepatocellular carcinoma: an overview of major findings from meta-analyses. Onco-target 2016; 7:34703–34751. https://doi.org/10.18632/oncotarget.9157
Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma. Cochrane Database Syst Rev 2017;3: CD011650. https://doi.org/10.18632/oncotarget.9157
Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection vs. percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300–307. https://doi.org/10.1016/j.jhep.2013.04.009
Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–12. https://doi.org/10.1097/SLA.0b013e3181efc656.
Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation vs. hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One 2014;9: e84484. https://doi.org/10.1371/journal.pone.0084484
Xin L, Wang Y, Gong J. Percutaneous radiofrequency ablation versus surgical resection for the treatment of small hepatic carcinoma: a meta-analysis. Am J Cancer Prev 2016;4:13–17. https://doi.org/10.12691/ajcp-4-1-3
Yu SCH, Hui EP, Wong J, et al. Transarterial ethanol ablation of hepatocellular carcinoma with lipiodol-ethanol mixture: phase II Study. J Vasc Interv Radiol. 2008;19:95–103. https://doi.org/10.1016/j.jvir.2007.08.038.
Yu SC, Hui JW, Hui EP, et al. Embolization efficacy and treatment effectiveness of transarterial therapy for unresectable hepatocellular carcinoma: a case-controlled comparison of transarterial ethanol ablation with lipiodol-ethanol mixture versus transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2009;20:352–9. https://doi.org/10.1016/j.jvir.2008.12.407.
Yu SC, Hui EP, Tang P, et al. Transarterial Ethanol Ablation for unresectable hepatocellular carcinoma: analysis of clinical and tumor outcomes. J Vasc Interv Radiol. 2016;27:639–649. https://doi.org/10.1016/j.jvir.2015.11.032
Yu SC, Hui JW, Hui EP, et al. Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology. 2014;270:607–20. https://doi.org/10.1148/radiol.13130498.
Yu SCH, Hui JWY, Chong CCN, et al. Transarterial ethanol ablation for small hepatocellular carcinoma (≤3 cm): A comparative study versus radiofrequency ablation. Cardiovasc Intervent Radiol. 2020;43:732–9. https://doi.org/10.1007/s00270-020-02426-4.
Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–8. https://doi.org/10.1200/JCO.2014.57.9151.
Wagener G. Assessment of hepatic function, operative candidacy, and medical management after liver resection in the patient with underlying liver disease. Semin Liver Dis. 2013;33:204–12. https://doi.org/10.1055/s-0033-1351777.
Cescon M, Colecchia A, Cucchetti A, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg 2012; 256:706–12–3. https://doi.org/10.1097/SLA.0000000000000394
Transarterial ethanol ablation of cirrhotic liver with lipiodol-ethanol mixture: safety and efficacy study in rats. Investig Radiol. 2006;41(8):609–617. https://doi.org/10.1097/01.rli.0000223884.05289.c3.
Mechanism and natural course of tumor involution in hepatocellular carcinoma following transarterial ethanol ablation. Cardiovasc Intervent Radiol. 2016;39(8):1136–1143. dx.doi.org/https://doi.org/10.1007/s00270-016-1360-z
Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28:1432–7. https://doi.org/10.1016/j.jvir.2017.06.019.
Chong CC, Lee KF, Ip PC, et al. Pre-operative predictors of post-hepatectomy recurrence of hepatocellular carcinoma: can we predict earlier? Surgeon. 2012;10:260–6.
Toyoda H, Lai PB, O’Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114:744–50. https://doi.org/10.1038/bjc.2016.33.
Chong CCN, Chan AWH, Wong J, et al. Albumin-bilirubin grade predicts the outcomes of liver resection versus radiofrequency ablation for very early/early stage of hepatocellular carcinoma. The Surgeon. 2018;16:163–70. https://doi.org/10.1016/j.surge.2017.07.003.
Chong CCN, Lee KF, Chu CM, et al. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: application of ALBI score to patient selection. HPB (Oxford). 2018;20:546–54. https://doi.org/10.1016/j.hpb.2017.12.001.
Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. https://doi.org/10.1016/S1470-2045(08)70284-5.
Chan AWH, Chong CCN, Mo FKF, et al. Applicability of albumin-bilirubin-based Japan integrated staging score in hepatitis B-associated hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1766–72. https://doi.org/10.1111/jgh.13339.
Chan AWH, Kumada T, Toyoda H, et al. Integration of albumin–bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1300–6. https://doi.org/10.1111/jgh.13291.
Miyayama S, Yamashiro M, Hashimoto M, et al. Comparison of local control in Transcatheter Arterial Chemoembolization of hepatocellular carcinoma ≤6 cm with or without intraprocedural monitoring of the embolized area using Cone-Beam Computed Tomography. Cardiovasc Intervent Radiol. 2014;37:388–95. https://doi.org/10.1007/s00270-013-0667-2.
Acknowledgement
The authors would like to thank Miss. Tiffany Lau and Mr. Lee Kwok Tung for their help in this study.
Funding
This study was funded by the Vascular and Interventional Radiology Foundation. The funding body had not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study formal consent is not required.
This study was approved by Institutional Review Board.
Informed Consent
This study has obtained IRB approval from (indicate the relevant board), and the need for informed consent was waived.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, S.C.H., Hui, J.W.Y., Chong, C.C.N. et al. Comparison of Survival Outcomes in Transarterial Ethanol Ablation and Liver Resection for Solitary Hepatocellular Carcinoma ≤ 5 cm in Patients Stratified by Liver Function. Cardiovasc Intervent Radiol 45, 315–327 (2022). https://doi.org/10.1007/s00270-021-02768-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02768-7